41 molecular orbital diagram for he2
Help constructing the molecular orbital diagram for He2^2+ Shape of a protein predicted by two different AI models (ESMFold on the left, AlphaFold on the right) 187. 16. r/cursed_chemistry. Join. Molecular orbital - Wikipedia In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule.This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms atomic orbital and molecular orbital were introduced by Robert S. Mulliken in 1932 to mean one-electron ...
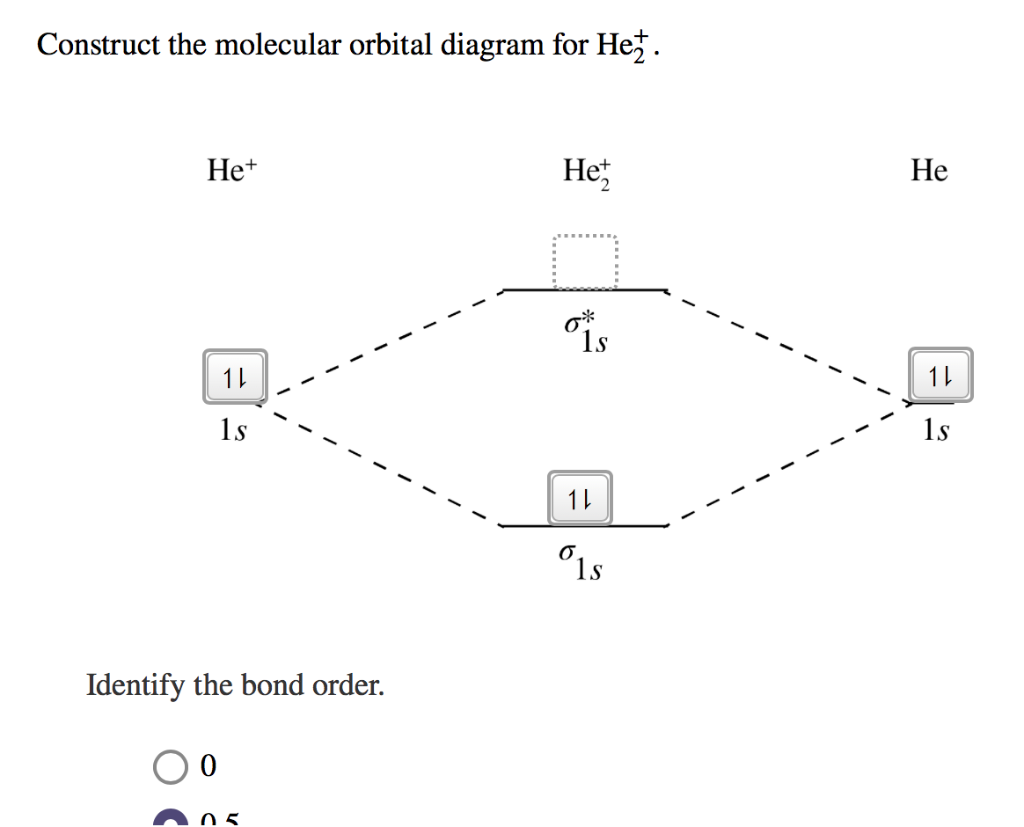
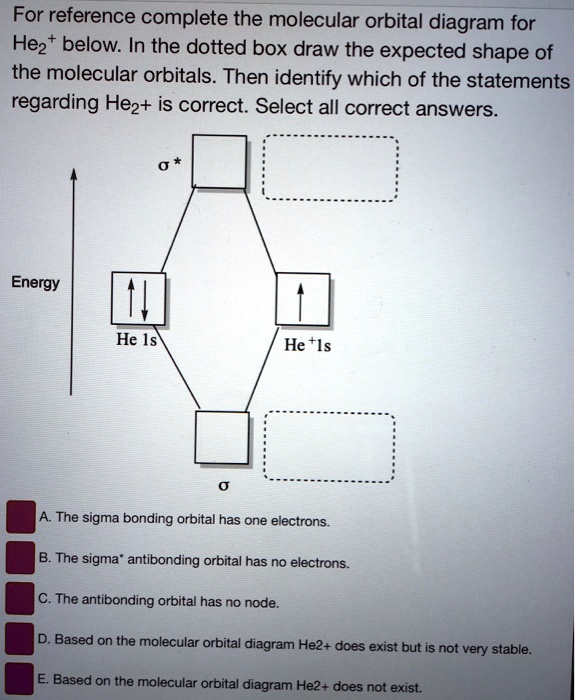
Solved Construct the molecular orbital diagram for | Chegg.com You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Construct the molecular orbital diagram for He2^+2 Identify the bond order. Show transcribed image text Expert Answer 100% (13 ratings) Answer : Number of electron : He = 2 He+ = 1 He cation dona … View the full answer
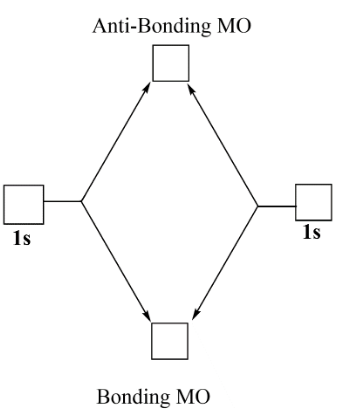
Molecular orbital diagram for he2
Draw the molecular orbital diagram of N2 and calculate the bond order. Q. Draw the molecular orbital diagram of N 2, N + 2 N − 2. Write their electronic configuration, find the bond order and predict their magnetic behaviour. Arrange the above in increasing order of bond length. Q. Give the Molecular Orbital Energy diagram of a) N 2 and b) O2. Calculate the respective bond order. Calculate the bond order of He2+ - BYJU'S Calculate the bond order of He2+ Molecular orbital correlation diagrams for He2, He2+, N2, N2+, CO, and ... Molecular orbital correlation diagrams for He2, He2+, N2, N2+, CO, and CO+. After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide molecules and some of their positive ions for the range of internuclear distances from about 1.5 times equilibrium down to 0.01 ...
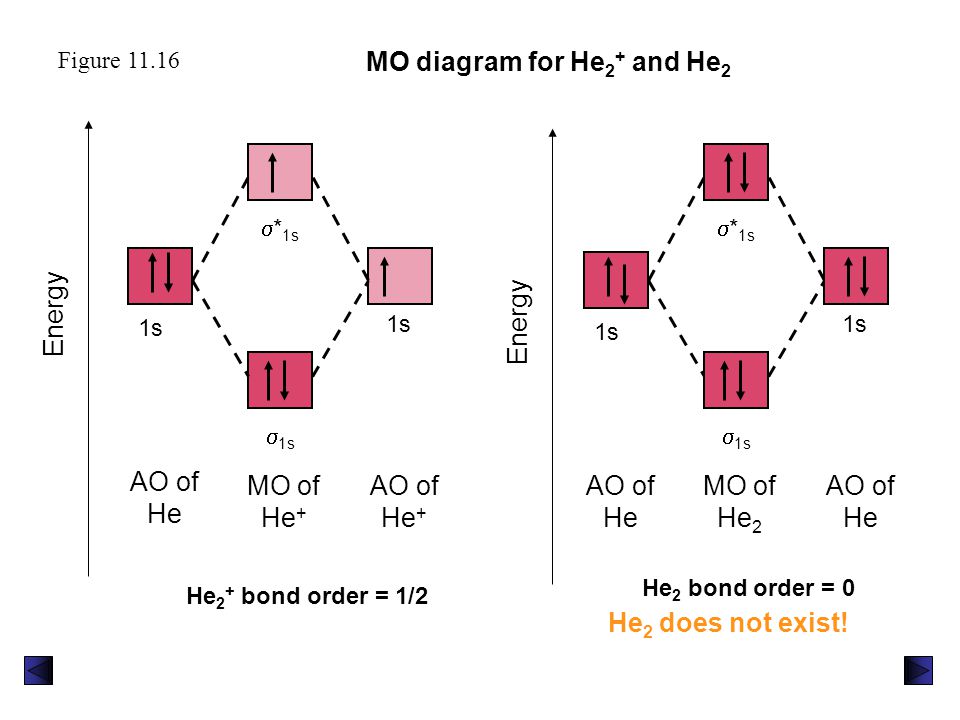
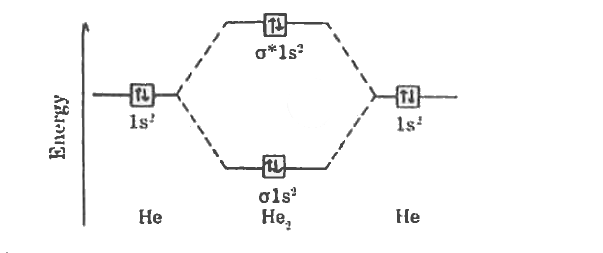
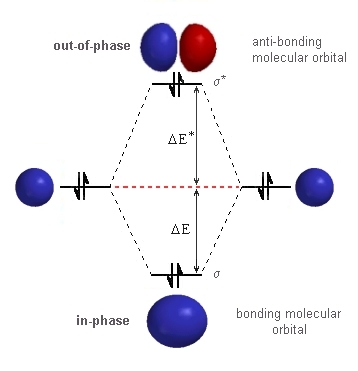
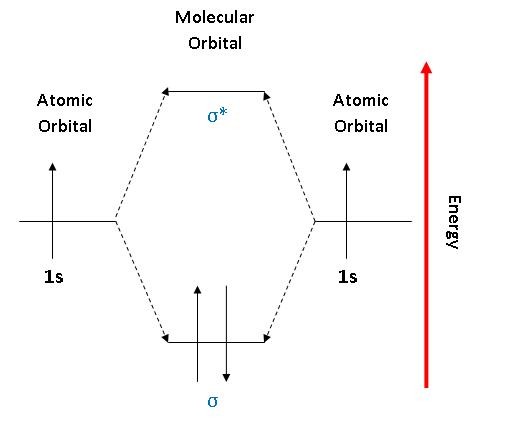
Molecular orbital diagram for he2. Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular… 7.7 Molecular Orbital Theory - Chemistry Fundamentals molecular orbital diagram ( Figure 7.7.9 ). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center. Why He2 molecule does not exist? Explain by MOT. Solution Verified by Toppr Electronic configuration of He is 1s 2. Molecular Orbital Diagram for He 2 is (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) = 22−2=0 ∴He 2 bond order is 0. There is no bond existing between atoms of He 2. So He 2 does not exist. How to Make the Molecular Orbital Diagram for He2: Does the Molecule ... The bond order of He2 is calculated and the meaning of this number ... This video discusses how to draw the molecular orbital (MO) diagram for the He2 molecule. The bond order of He2 is...
Mo Diagram He2 The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding. Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... 8 - Drawing Molecular Orbital Diagrams — Flux Science Homonuclear molecular orbitals are formed between two elements that are the same, meaning that they are naturally symmetrical and will perfectly overlap. However, before we fill out this diagram, compare this MOD to the one above, particularly in the 2p region. They're pretty similar, except with their sigma and pi bonding molecular orbitals. What is the MOED of $H{e_2}$ molecule? - Vedantu It is given as: B o n d o r d e r = 1 2 [ N b − N a] And we got from the diagram that Helium has two electrons in its bonding molecular orbital and two electrons in its anti-bonding molecular orbital. So its bond order will be: B o n d o r d e r = 1 2 [ 2 − 2] = 0
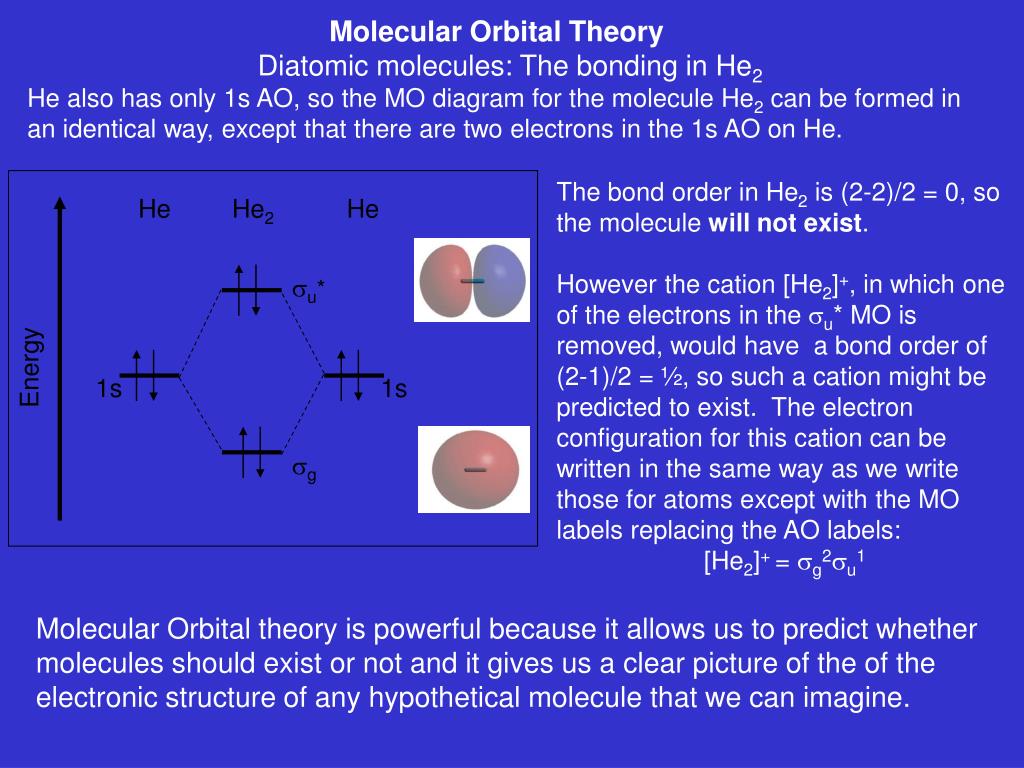
chemical bonding - Molecular orbitals of H2 and He2 | Britannica The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2. Molecular Orbital Diagram He2 In He2 molecule, Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total number of electrons available are 4. Molecular. He2 is not possible. He MO Diagram. Eg: He + H; same mixing as above. Three electrons, two in sigma, one in sigma*. molecular orbital diagram for he2 He2 orbital molecular diagram mo bond ... orbital molecular electrons ion atomic he2 Molecular orbital diagram bond order h2 construct mo identify then he2 stable exist bonding schematron theory orbitals. Orbital molecular diagram f2 label each below mo. Molecular orbital energy-level diagram Category: Diagrams Post navigation Previous post: 5.7 vortec belt diagram 5.3 belt diagram Why is He2 not a stable molecule? - CHEMISTRY COMMUNITY To answer the question, you must construct a molecular orbital (MO) diagram for the hypothetical He 2 molecule. The σ 1s bonding and antibonding orbitals will be full. Calculating the bond order results in 0. In other words, no bond can be sustained between two He atoms according to MO theory. Top.
Molecular Orbital Diagram For He2 The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = .
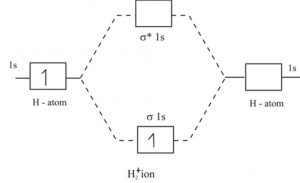
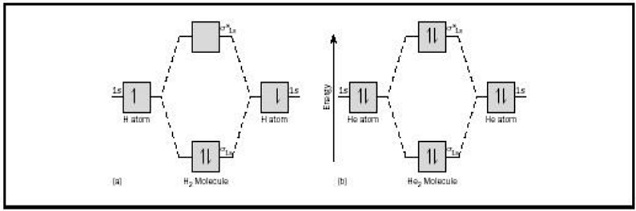
2.3b: MO theory of bonding in H₂⁺ - Chemistry LibreTexts Figure 3: Schematic represenation of antibonding molecular orbital σ* (1s) Note that there is a nodal plane in the anti-bonding MO. Bond order Bond order = 1/2 (#e- in bonding MO - #e- in antibonding MO) For H 2, bond order = 1/2 (2-0) = 1, which means H 2 has only one bond. The antibonding orbital is empty. Thus, H 2 is a stable molecule.
Molecular Orbital Theory | Boundless Chemistry | | Course Hero The bonding diagram for the hypothetical molecule He2.: Notice the two electrons occupying the antibonding orbital, which explains why the He 2 molecule does not exist. ... Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. ...
Do He2, He2(+), He2(2+) exist, stable? (Molecular Orbital Theory ... In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the... AboutPressCopyrightContact...
How do I calculate the bond order for H2- and H2+? | Socratic Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals. If you calculate their bond order, you get: BOH+ 2 = 1 2(Bonding − ...
Molecular orbital energy level diagrams -Hydrogen, Hypothetical ... He2 : (s1s)2 (s*1s)2. The molecular orbital energy level diagram of He2 (hypothetical) is given in Fig. Here, Nb = 2 and Na = 2. Bond order = Nb - Na / 2 = 2 - 2 / 2 = 0. As the bond order for He2 comes out to be zero, this molecule does not exist. 3.
9.7: Molecular Orbitals - Chemistry LibreTexts For the He2 molecule, each He has a full 1 s orbital. This means that sigma 1s and sigma * 1s orbital are full. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron. The single electron occupies the σ 1s bonding molecular orbital, giving a (σ 1s) 1 electron configuration.
Construct the molecular orbital diagram for he2 - AnswerData Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons. Answer General guidance Concepts and reason Bond order is the number, which indicates the total number of bonds present between two atoms. Bond order describes the bond strength of the molecule;
Free Learn Diagram - Page 12 of 298 - Get Free Diagram and Flowchart mo diagram use n2 orbital molecular answer below c2 bond questions order provided following. Molecular Orbital Diagram For Ne2. diagramweb.net. ne2 orbital he2 molecule orbitals diagrams monoxide. 34 No+ Molecular Orbital Diagram - Wiring Diagram Database. kovodym.blogspot.com. diagram molecular orbital reactivity understanding chemical ...
Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
h2 molecular orbital diagram Molecular orbital diagram for he2 We have 9 Images about Molecular Orbital Diagram For He2 like Molecular Orbital Diagram For He2, Construct The Molecular Orbital Diagram For He2 And Then Identify The and also Molecular Orbital Diagram For He2. Here you go: Molecular Orbital Diagram For He2 diagramweb.net
Molecular orbital correlation diagrams for He2, He2+, N2, N2+, CO, and ... Molecular orbital correlation diagrams for He2, He2+, N2, N2+, CO, and CO+. After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide molecules and some of their positive ions for the range of internuclear distances from about 1.5 times equilibrium down to 0.01 ...
Calculate the bond order of He2+ - BYJU'S Calculate the bond order of He2+
Draw the molecular orbital diagram of N2 and calculate the bond order. Q. Draw the molecular orbital diagram of N 2, N + 2 N − 2. Write their electronic configuration, find the bond order and predict their magnetic behaviour. Arrange the above in increasing order of bond length. Q. Give the Molecular Orbital Energy diagram of a) N 2 and b) O2. Calculate the respective bond order.






















0 Response to "41 molecular orbital diagram for he2"
Post a Comment