41 free energy reaction coordinate diagram
Chemical kinetics - Wikipedia Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate.Chemical kinetics includes investigations of how experimental … 5.3. Reaction coordinate diagrams - Lumen Learning Energy diagrams for these processes will often plot the enthalpy (H) instead of Free Energy for simplicity.The standard Gibbs Free Energy change for a reaction can be related to the reaction's equilibrium constant (K eq) by a simple equation:ΔG˚ = -RT ln K eq where: K eq = [product] / [reactant] at equilibrium
Physics Classroom Concept Checkers were built to coordinate with the Physics Interactives section of our website. Relying upon our Task Tracker tool to deliver questions, check answers, and track student progress, a Concept Checker is designed to coordinate with an interactive simulation. Use it as a follow-up check to understanding after exploring the simulation.

Free energy reaction coordinate diagram
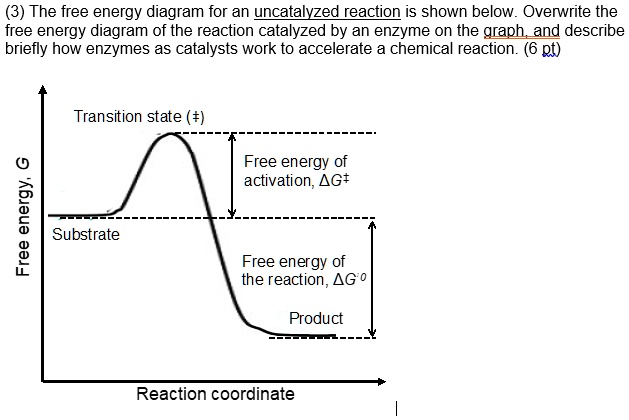
6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this: The Physics Classroom The Physics Classroom serves students, teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional. Written by teachers for teachers and students, The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers. Energy profile (chemistry) - Wikipedia Figure 1: Reaction Coordinate Diagram: Starting material or reactant A convert to product C via the transition state B, with the help of activation energy ΔG≠, after which chemical energy ΔG° is released Qualitatively, the reaction coordinate diagrams (one-dimensional energy surfaces) have numerous applications.
Free energy reaction coordinate diagram. 04.02 Reaction Coordinate Diagrams and Stability Trends 18.5K subscribers General structure of a reaction coordinate diagram, including transition states and intermediates. Overall free energy change and activation energy. Definition of a... › class › newtlawsIdentifying Interaction Force Pairs - Physics Classroom Forces always come in pairs - known as "action-reaction force pairs." Identifying and describing action-reaction force pairs is a simple matter of identifying the two interacting objects and making two statements describing who is pushing on whom and in what direction. For example, consider the interaction between a baseball bat and a baseball. Home | ExploreLearning Solve the math fact fluency problem. Adaptive and individualized, Reflex is the most effective and fun system for mastering basic facts in addition, subtraction, multiplication and division for grades 2+. ClassLink ClassLink
Energy/Reaction Coordinate Diagrams Thermodynamics, Kinetics - SlideServe A Reaction Coordinate (Energy) Diagram. Gibbs standard free energy change (DGo) Enthalphy (DHo): the heat given off or absorbed during a reaction Entropy (DSo): a measure of freedom of motion Thermodynamic Quantities DGo = DHo - TDSo DG,DH,DS,DE are state functions E = q + w H = qp If DSo is small, compared to DHo, then DGo ≈DHo Reaction Coordinates in Potential Energy Diagrams Reaction potential energy diagrams are graphs that show the energy of a process as a function of the extent to which that process has occurred. As these are graphs showing mathematical functions, there must be a numerical coordinate axis that shows the independent variable. 5.6: Reaction Energy Diagrams and Transition States In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this: Practice: Reaction Coordinate Energy Diagrams - YouTube In this video, Dr. Norris goes over practice problems in interpreting reaction energy coordinate diagrams.
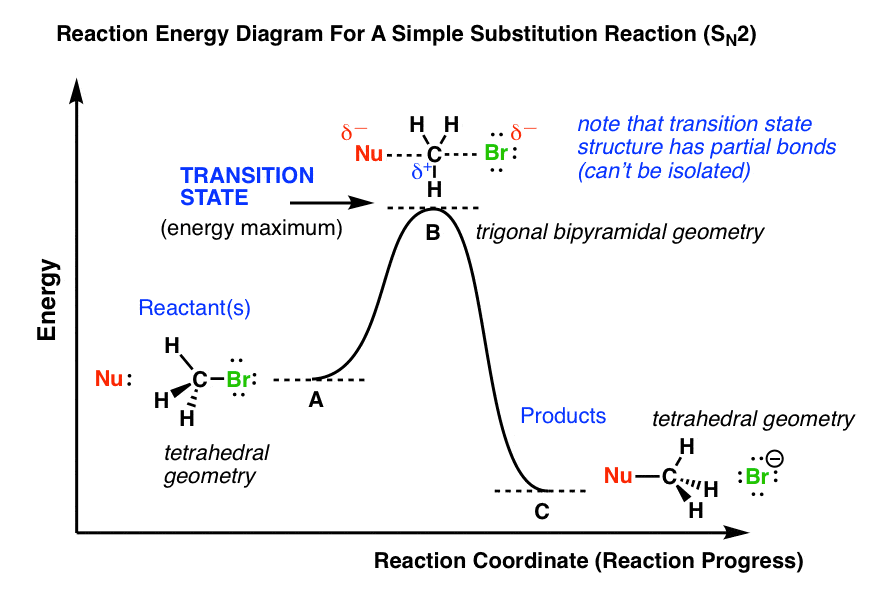
Energy Diagram - thechemistrynotes.com Free-energy diagrams, also known as reaction coordinate diagrams, are graphs that portray the energy changes during a chemical reaction. The horizontal axis reaction coordinate represents how far the reaction has progressed structurally. Positions along the x-axis near the reactants have structures similar to the reactants. en.wikipedia.org › wiki › Rate-determining_stepRate-determining step - Wikipedia In a multistep reaction, the rate-determining step does not necessarily correspond to the highest Gibbs energy on the reaction coordinate diagram. [8] [6] If there is a reaction intermediate whose energy is lower than the initial reactants, then the activation energy needed to pass through any subsequent transition state depends on the Gibbs ... en.wikipedia.org › wiki › Chemical_kineticsChemical kinetics - Wikipedia Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Reaction coordinate - Wikipedia Reaction coordinate is distinct from extent of reaction, a different parameter of reaction progress, which is a measure of the composition of the reaction system. (Free) energy is often plotted against reaction coordinate(s) to demonstrate in some schematic form the potential energy profile (an intersection of a potential energy surface ...
E1 Reaction Coordinate Energy Diagram - YouTube presents: E1 Reaction Coordinate Energy Diagram with step by step mechanism, transition states and intermediates📺Watch Next...
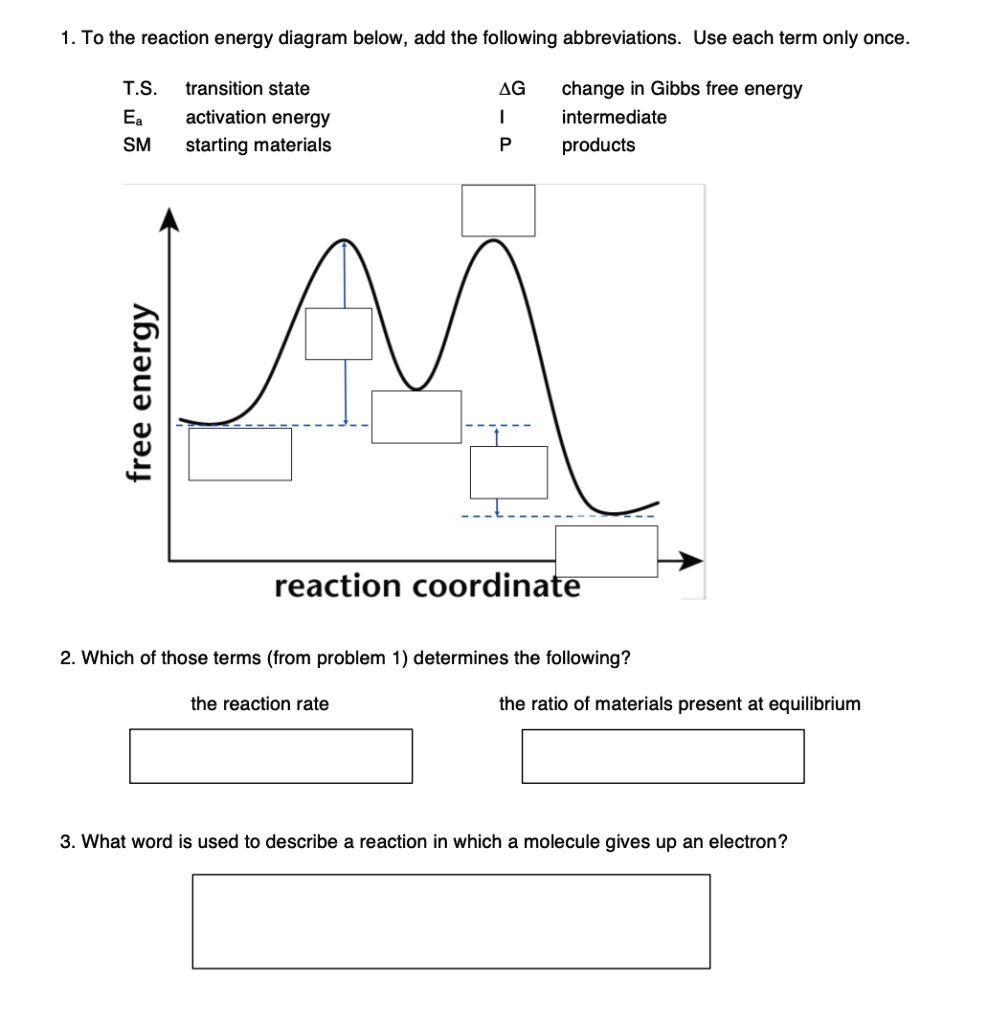
Reaction Coordinate Diagram - An Overview of Reaction ... - BYJUS A reaction coordinate diagram is a graph that shows the relationship between energy and reaction progress. Explaining Reaction Coordinate Diagram Let's begin with the letter 'A.' This is a picture of the reactants before the reaction. Energy is contributed to the reaction as it occurs, and this energy is frequently in the form of heat.
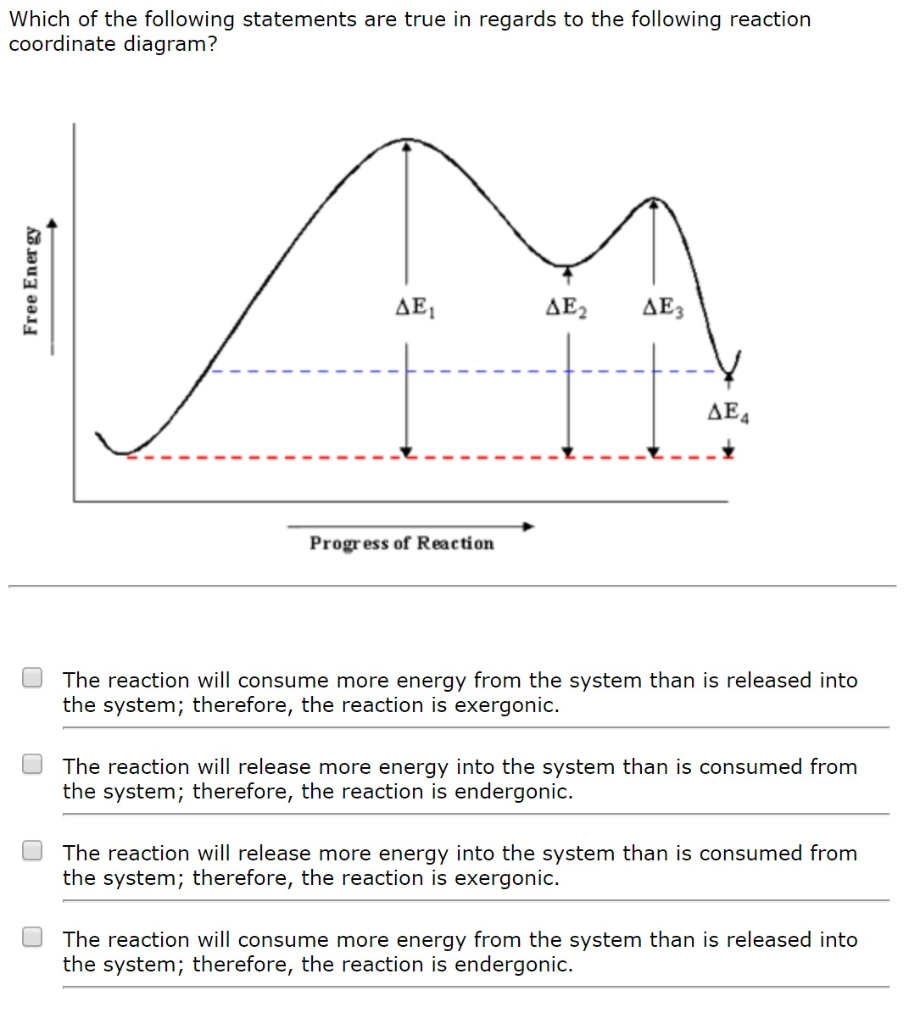
Solved 76 Question (3 points) Use the free energy versus | Chegg.com Question: 76 Question (3 points) Use the free energy versus reaction coordinate diagram to answer the three questions below. B С free energy A reactants E products reaction coordinate K1 76mon Part 1 (1 pt) See Periodic Table See Hint How many transition states are there? transition states Part 2 (1 pt) Which of the following represents AGfor ...
Identifying Interaction Force Pairs - Physics Classroom According to Newton's third law, for every action force there is an equal (in size) and opposite (in direction) reaction force.Forces always come in pairs - known as "action-reaction force pairs." Identifying and describing action-reaction force pairs is a simple matter of identifying the two interacting objects and making two statements describing who is pushing on whom and in what …
en.wikipedia.org › wiki › Reaction_coordinateReaction coordinate - Wikipedia Reaction coordinate is distinct from extent of reaction, a different parameter of reaction progress, which is a measure of the composition of the reaction system. (Free) energy is often plotted against reaction coordinate(s) to demonstrate in some schematic form the potential energy profile (an intersection of a potential energy surface ...
en.wikipedia.org › wiki › Gibbs_free_energyGibbs free energy - Wikipedia Δ r G, Gibbs free energy change per mole of reaction, Δ r G°, Gibbs free energy change per mole of reaction for unmixed reactants and products at standard conditions (i.e. 298 K, 100 kPa, 1 M of each reactant and product), R, gas constant, T, absolute temperature, ln, natural logarithm, Q r, reaction quotient (unitless),
| ExploreLearning Solve the math fact fluency problem. Adaptive and individualized, Reflex is the most effective and fun system for mastering basic facts in addition, subtraction, multiplication and division for grades 2+.
Rate-determining step - Wikipedia In a multistep reaction, the rate-determining step does not necessarily correspond to the highest Gibbs energy on the reaction coordinate diagram. [8] [6] If there is a reaction intermediate whose energy is lower than the initial reactants, then the activation energy needed to pass through any subsequent transition state depends on the Gibbs ...
Energy Diagrams - Chemistry LibreTexts Energy diagrams for these processes will often plot the enthalpy (H) instead of Free Energy for simplicity. The standard Gibbs Free Energy change for a reaction can be related to the reaction's equilibrium constant (K eq) by a simple equation: Δ G˚ = -RT ln K eq where: K eq = [product] / [reactant] at equilibrium
5.3: Reaction coordinate diagrams - Chemistry LibreTexts Energy diagrams for these processes will often plot the enthalpy (H) instead of Free Energy for simplicity.The standard Gibbs Free Energy change for a reaction can be related to the reaction's equilibrium constant (K eq) by a simple equation:ΔG˚ = -RT ln K eq where: If you do the math, you see that a negative value for ΔG˚ rxn (an ...
Reaction Coordinate Diagrams | Exothermic vs. Endothermic Graphs ... A reaction coordinate diagram illustrates the relationship between the energy movement in a chemical reaction and the progress of the reaction. The reactants are the substances to...
Gibbs free energy - Wikipedia According to the second law of thermodynamics, for systems reacting at fixed temperature and pressure without input of non-Pressure Volume (PV) work, there is a general natural tendency to achieve a minimum of the Gibbs free energy.. A quantitative measure of the favorability of a given reaction under these conditions is the change ΔG (sometimes written "delta G" or "dG") …
CNN 10 - CNN CNN 10 is an on-demand digital news show ideal for explanation seekers on the go or in the classroom.
Catalysis - Wikipedia Catalysis (/ k ə ˈ t æ l ə s ɪ s /) is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (/ ˈ k æ t əl ɪ s t /).Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are ...
Energy profile (chemistry) - Wikipedia Figure 1: Reaction Coordinate Diagram: Starting material or reactant A convert to product C via the transition state B, with the help of activation energy ΔG≠, after which chemical energy ΔG° is released Qualitatively, the reaction coordinate diagrams (one-dimensional energy surfaces) have numerous applications.
The Physics Classroom The Physics Classroom serves students, teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional. Written by teachers for teachers and students, The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers.
6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this:



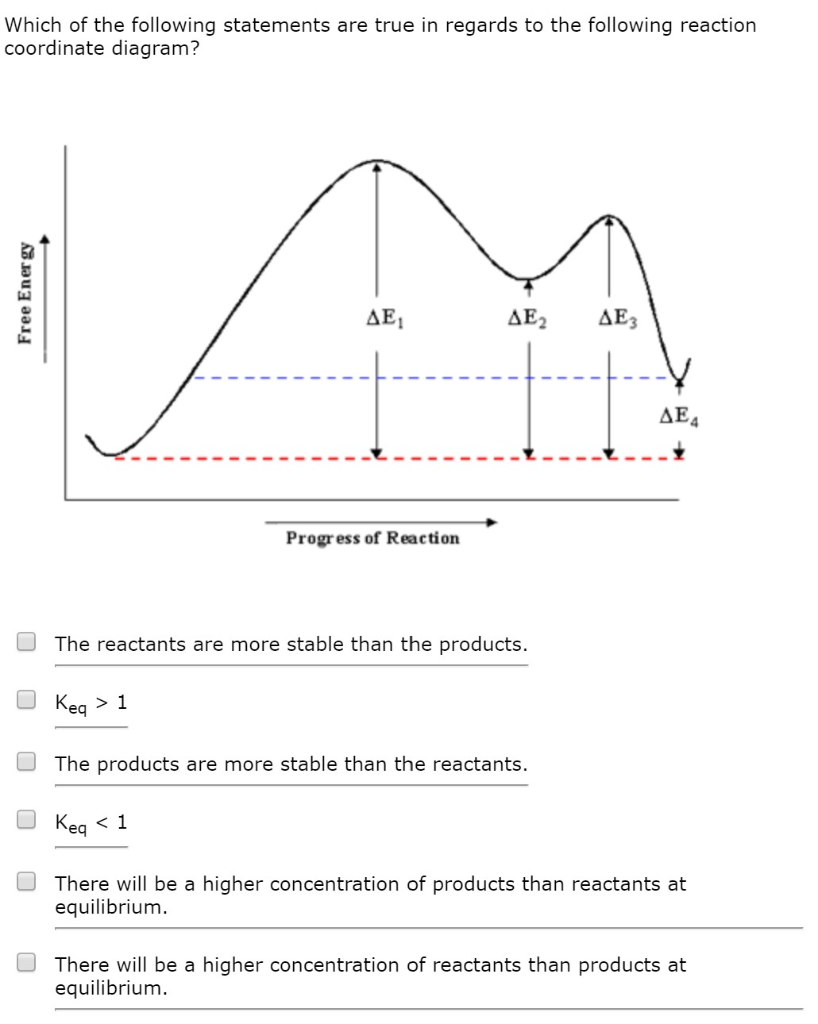
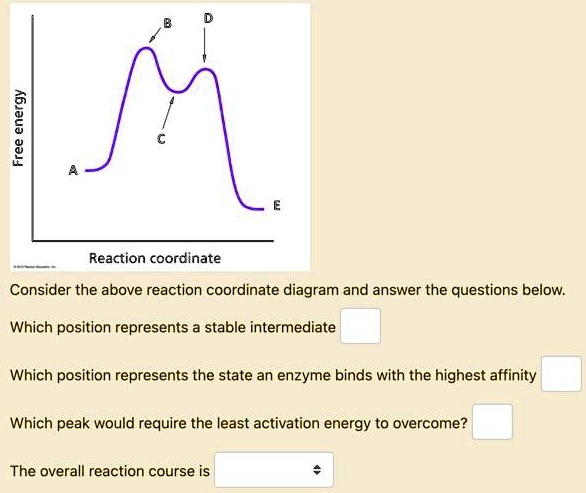


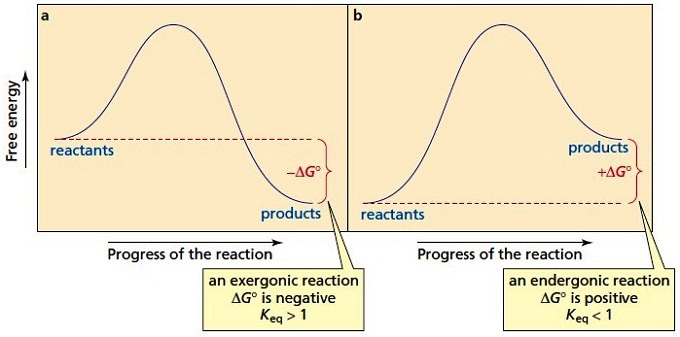













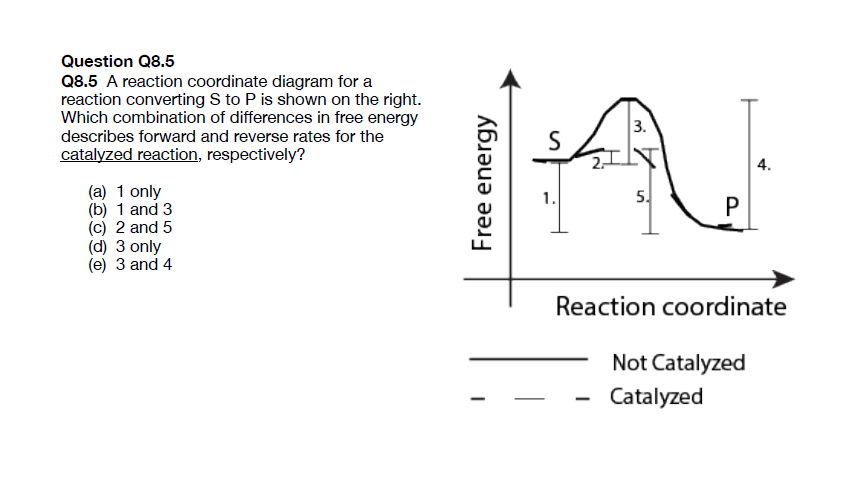

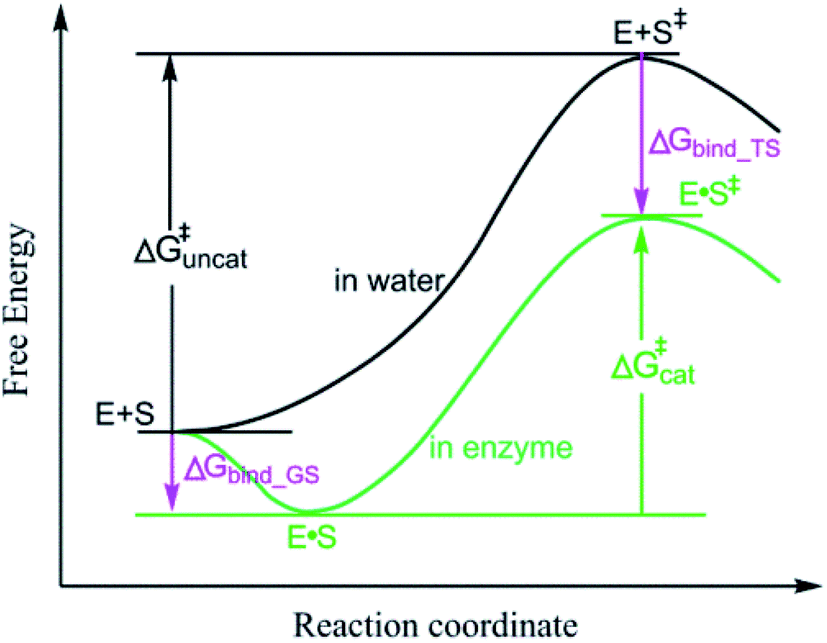
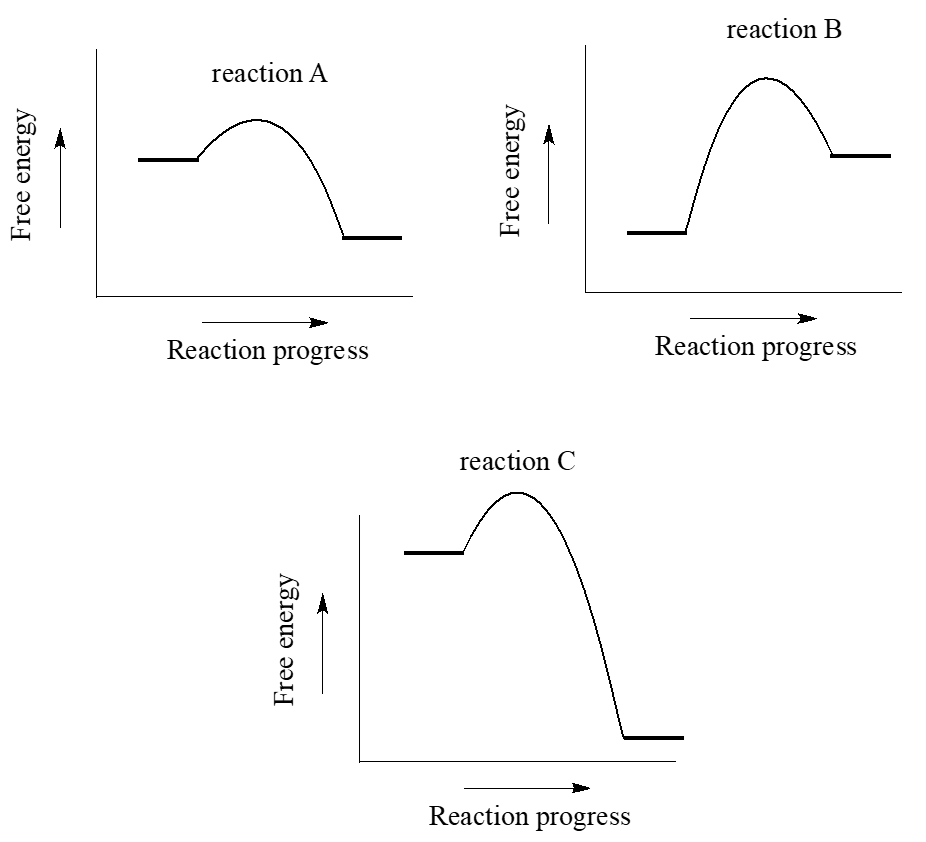



0 Response to "41 free energy reaction coordinate diagram"
Post a Comment