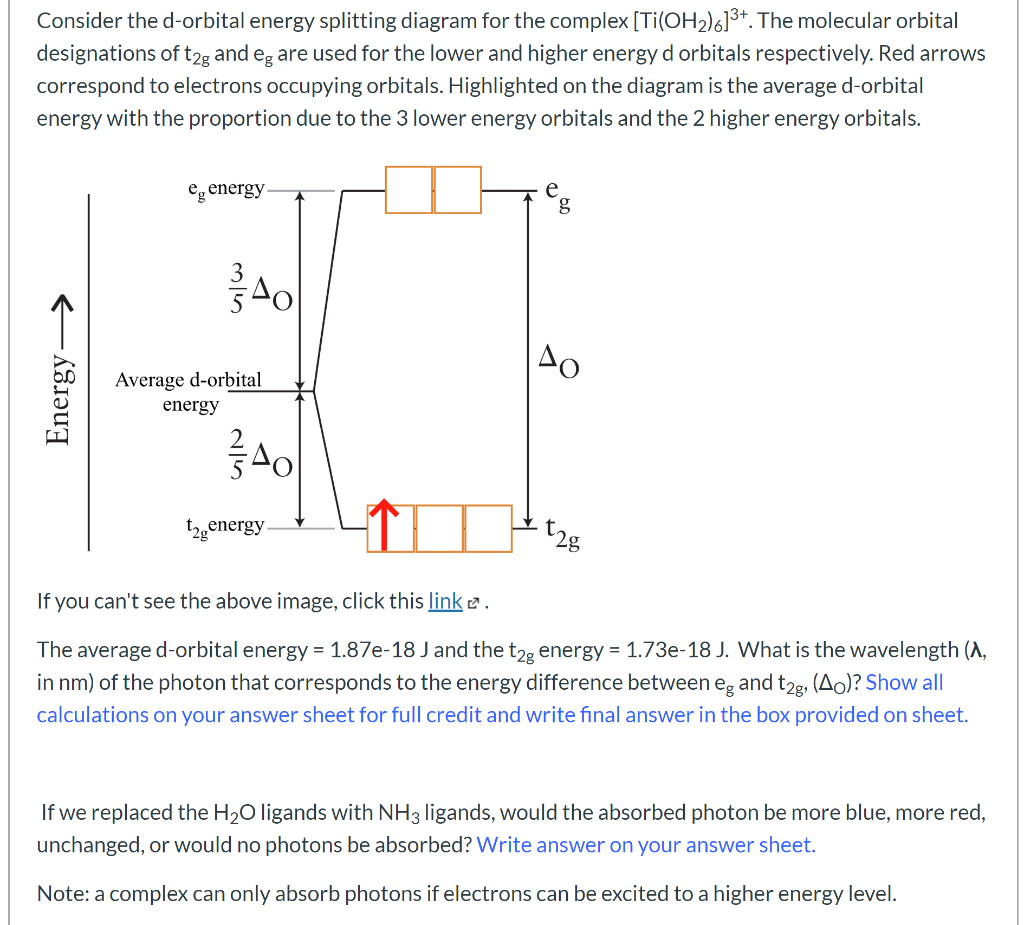
38 d orbital energy level diagram
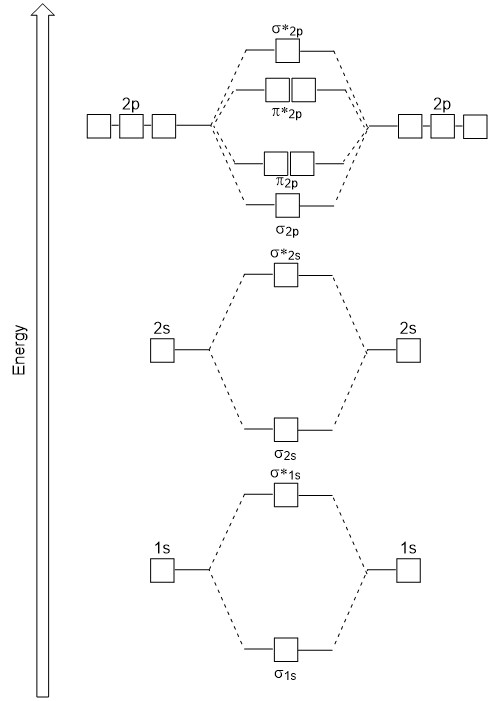
Lecture 3 Stable electronic configurations: MO Energy Level Diagrams Reviewed ... Ligand (σ- and π*) orbitals and metal d-orbitals are shown.) Simplified MO energy ... Phosphorus(P) electron configuration and orbital diagram The Aufbau method is to do electron configuration through the sub-energy level. The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. The energy of an orbital is calculated from the value of the principal quantum number ‘n ...
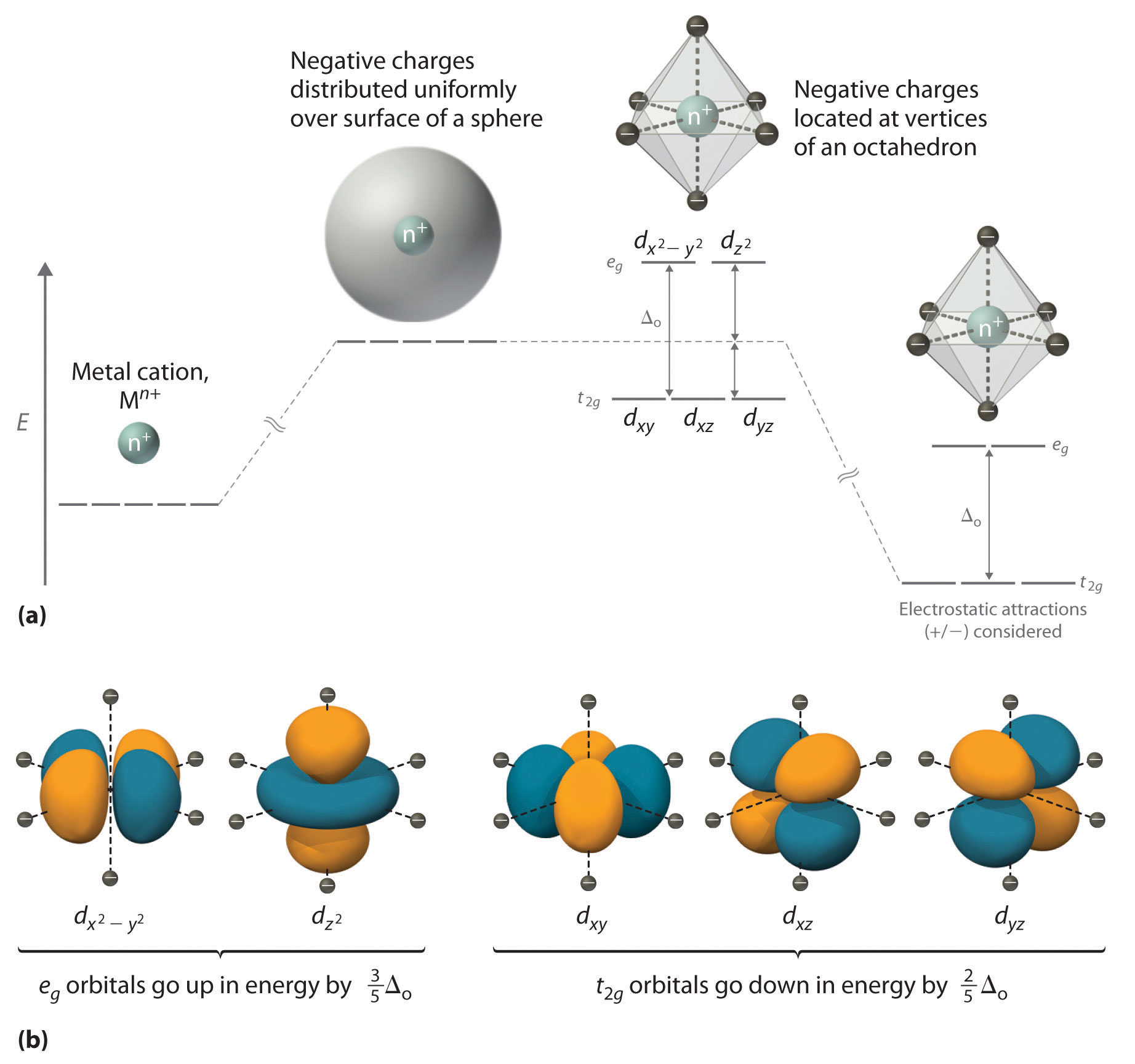
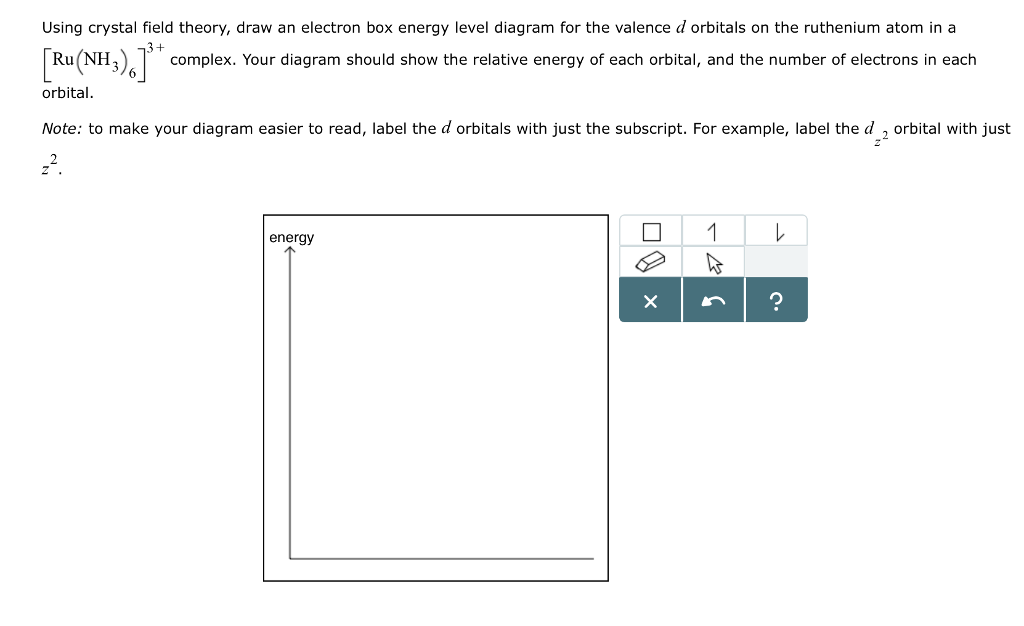
Crystal Field Theory - Chemistry LibreTexts May 6, 2021 ... Placing a charge of −1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies: the dx2−y2 and ...

D orbital energy level diagram
Energy level - Wikipedia The energy level of the bonding orbitals is lower, and the energy level of the antibonding orbitals is higher. For the bond in the molecule to be stable, the covalent bonding electrons occupy the lower energy bonding orbital, which may be signified by such symbols as σ or π depending on the situation. Prefrontal cortex - Wikipedia According to Striedter the PFC of humans can be delineated into two functionally, morphologically, and evolutionarily different regions: the ventromedial PFC (vmPFC) consisting of the ventral prefrontal cortex and the medial prefrontal cortex present in all mammals, and the lateral prefrontal cortex (LPFC), consisting of the dorsolateral prefrontal cortex and the ventrolateral prefrontal ... Oxygen(O) electron configuration and orbital diagram The Aufbau method is to do electron configuration through the sub-energy level. The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. The energy of an orbital is calculated from the value of the principal quantum number ‘n ...
D orbital energy level diagram. Answer the following question. Draw a qualitatively energy-level ... Draw a qualitatively energy-level diagram showing d-orbital splitting in the octahedral environment. Predict the number of unpaired electrons in the complex ... CHAPTER 5: MOLECULAR ORBITALS d. NO+. Bond order = 3 shortest bond (106 pm). NO Bond order = 2.5 ... The energy level diagrams for CH2 and BeH2 feature the same orbital interactions. One. D-orbital splitting diagrams - Berkeley Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral. Overwatch 2 reaches 25 million players, tripling Overwatch 1 ... Oct 14, 2022 · Following a bumpy launch week that saw frequent server trouble and bloated player queues, Blizzard has announced that over 25 million Overwatch 2 players have logged on in its first 10 days."Sinc
Draw an orbital energy-level diagram showing the configuration of ... The orbital diagram is produced in accordance with crystal field theory for any particular metal ion and its ligand. This method provides information on the d- ... Draw qualitatively energy-level diagram showing d-orbital splitting in ... Jun 27, 2022 ... Draw qualitatively energy-level diagram showing d-orbital splitting in the octahedral environment. Predict the number of unpaired electrons ... The qualitative d-orbital energy-splitting diagram for pentagonal... The single-crystal X-ray diffraction (SC-XRD) study reveals the pentagonal bipyramidal molecular structure of the [Os(CN)7]3− anion. The latter being... Cite. Earth - Wikipedia These dates change over time due to precession and other orbital factors, which follow cyclical patterns known as Milankovitch cycles. The changing Earth-Sun distance causes an increase of about 6.8% in solar energy reaching Earth at perihelion relative to aphelion.
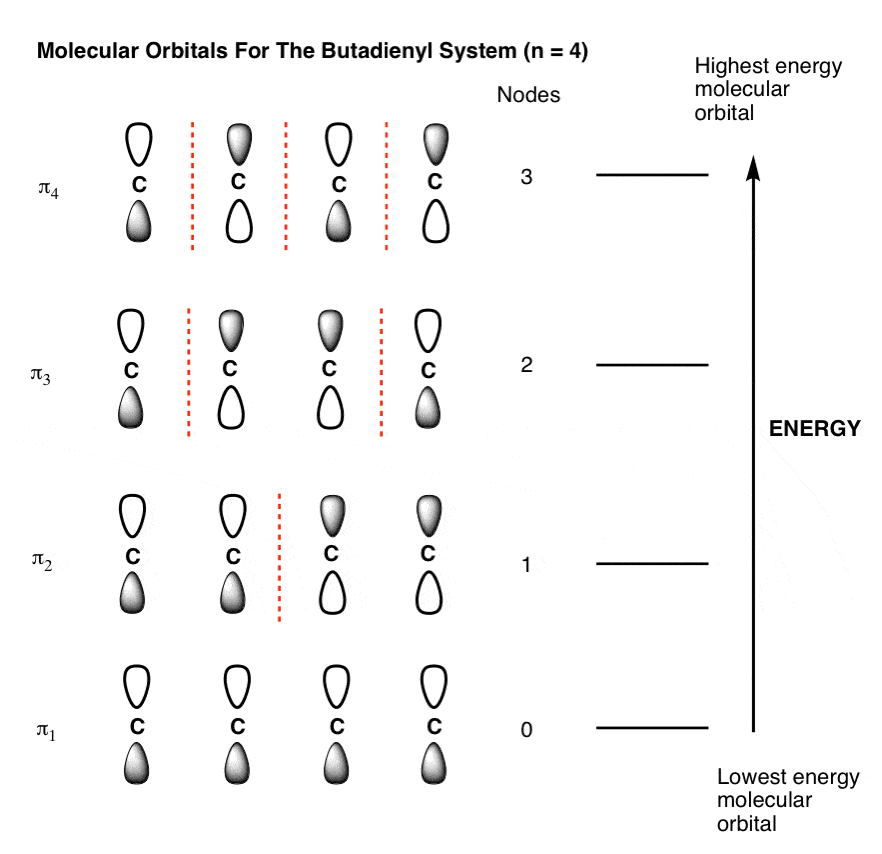
Energy level diagrams and electron orbitals - YouTube Nov 29, 2015 ... A look at the energy levels of electron orbitals. The video looks at electron pair repulsion and the different electron configuration of ... Energy of Orbitals - Calculating the Energy Level, Solved ... So the 4s orbital has a higher (n+l) value, thus has a higher orbital energy level. Ans: 3d . 2. Which of these orbitals has a higher orbital energy level 3d or 4p? The (n+l) value of 3d orbital is (3+2) = 5, and 4p orbital is (4+1)=5. Both have the same (n+l) value with 3d having a lower n-count; thus, it is weaker and has a lower orbital ... Category:Molecular orbitals energy levels - Wikimedia Commons Dec 19, 2016 ... Media in category "Molecular orbitals energy levels" ... MO diagram octahedral pi acceptor filled.jpg 507 × 536; 43 KB. Oxygen(O) electron configuration and orbital diagram The Aufbau method is to do electron configuration through the sub-energy level. The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. The energy of an orbital is calculated from the value of the principal quantum number ‘n ...
Prefrontal cortex - Wikipedia According to Striedter the PFC of humans can be delineated into two functionally, morphologically, and evolutionarily different regions: the ventromedial PFC (vmPFC) consisting of the ventral prefrontal cortex and the medial prefrontal cortex present in all mammals, and the lateral prefrontal cortex (LPFC), consisting of the dorsolateral prefrontal cortex and the ventrolateral prefrontal ...
Energy level - Wikipedia The energy level of the bonding orbitals is lower, and the energy level of the antibonding orbitals is higher. For the bond in the molecule to be stable, the covalent bonding electrons occupy the lower energy bonding orbital, which may be signified by such symbols as σ or π depending on the situation.


























0 Response to "38 d orbital energy level diagram"
Post a Comment