45 the diagram represents a spontaneous reaction. use the diagram to answer the questions below.
The diagram below represents a spontaneous reaction (δg° The diagram below represents a spontaneous reaction (deltaG degree Answer General guidance Concepts and reason In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous reaction. What is a spontaneous reaction? - OneClass Get the detailed answer: What is a spontaneous reaction? From the values of delta H and delta S, predict which of the following reactions would be spontaneous at 28 degree C: reaction A: delta H = 10.5 kJ/mol, delte S = 30.0 J/K. mol: spontaneous nonspontaneous impossible to tell reaction B: delta H = 1.8 kJ/mol, delta S = -113 J/K middot mol, spontaneous nonspontaneous impossible to tell If ...
Question 1 Which of these diagrams indicate(s) a spontaneous... Question 11. You performed a reaction that occurred much too quickly and nearly injured a classmate. You need to perform the reaction again. Identify ways that you can s low the rate of a reaction. Select either increase or decrease next to each variable to indicate what choice you would make to slow reaction rate. Temperature. Pressure. Volume.
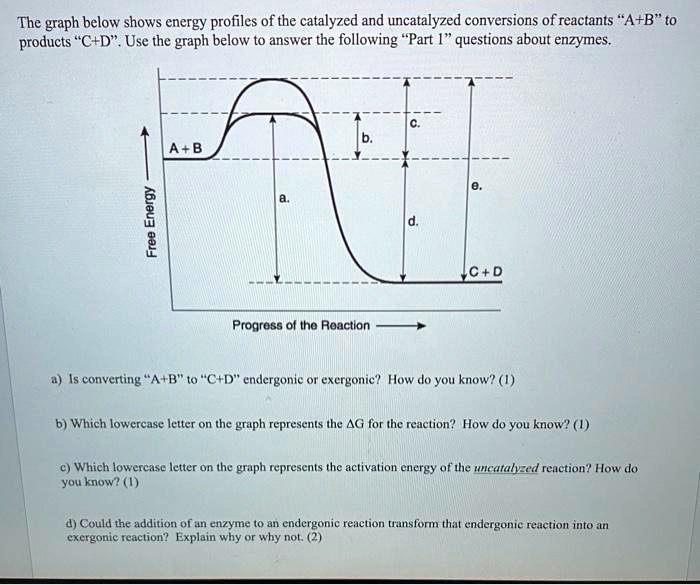
The diagram represents a spontaneous reaction. use the diagram to answer the questions below.
Free Energy Practice Exam Questions Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like Refer to the free energy diagrams below to answer the following questions. You may assume that the y-axis is the same and directly comparable for all four reactions., 1.Reaction C is endergonic: -True -False, 2. Reaction A should occur at a faster rate than Reaction D: -True -False and more. Which equation represents a spontaneous reaction? A) Ca + Ba2+ ⇒ Ca2 ... The reaction involves the interaction between the reactant(s) to form the product(s). The fourth equation involving manganese and nickel is a spontaneous reaction. Thus, option D is correct.. What is a spontaneous reaction? A spontaneous reaction is the product formation at the same time the reactants are changing. It generally involves the release of the energy and does not need external energy. the diagram below represents a spontaneous reaction (δg° Spontaneous ... If you are searching about January 2016 you've visit to the right place. We have 9 Pics about January 2016 like 32 The Diagram Below Represents A Spontaneous Reaction δg - Wiring, The Diagram Below Represents A Spontaneous Reaction δg - Atkinsjewelry and also Spontaneous Reaction - YouTube. Here it is: January 2016 wiringdiagram99.blogspot.com
The diagram represents a spontaneous reaction. use the diagram to answer the questions below.. 150-1 The diagram represents a spontaneou... - Physical Chemistry Physical Chemistry. Chemical kinetics Solutions. Under certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0015 M-s -1 2 HI(g) -H(g) +12 (8) Suppose a 4.0 L flask is charged under these conditions with 400. mmol of hydrogen iodide. Which diagram represents the potential energy of an exothermic reaction ... use the potential energy diagram to answer the questions below:. given the potential energy diagram for a reversible chemical reaction In accordance with Hammond's postulate, exothermic reactions tend to have _____. 991447. expand. the diagram below represents a spontaneous reaction (δg° Spontaneous ... If you are searching about January 2016 you've visit to the right place. We have 9 Pics about January 2016 like 32 The Diagram Below Represents A Spontaneous Reaction δg - Wiring, The Diagram Below Represents A Spontaneous Reaction δg - Atkinsjewelry and also Spontaneous Reaction - YouTube. Here it is: January 2016 wiringdiagram99.blogspot.com Which equation represents a spontaneous reaction? A) Ca + Ba2+ ⇒ Ca2 ... The reaction involves the interaction between the reactant(s) to form the product(s). The fourth equation involving manganese and nickel is a spontaneous reaction. Thus, option D is correct.. What is a spontaneous reaction? A spontaneous reaction is the product formation at the same time the reactants are changing. It generally involves the release of the energy and does not need external energy.
Free Energy Practice Exam Questions Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like Refer to the free energy diagrams below to answer the following questions. You may assume that the y-axis is the same and directly comparable for all four reactions., 1.Reaction C is endergonic: -True -False, 2. Reaction A should occur at a faster rate than Reaction D: -True -False and more.

0 Response to "45 the diagram represents a spontaneous reaction. use the diagram to answer the questions below."
Post a Comment