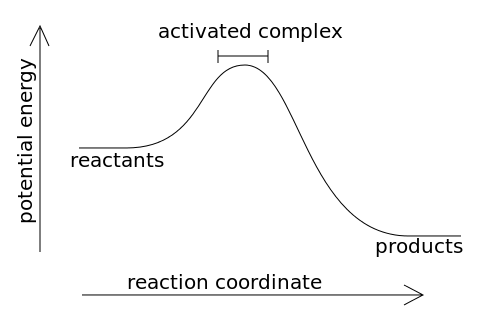
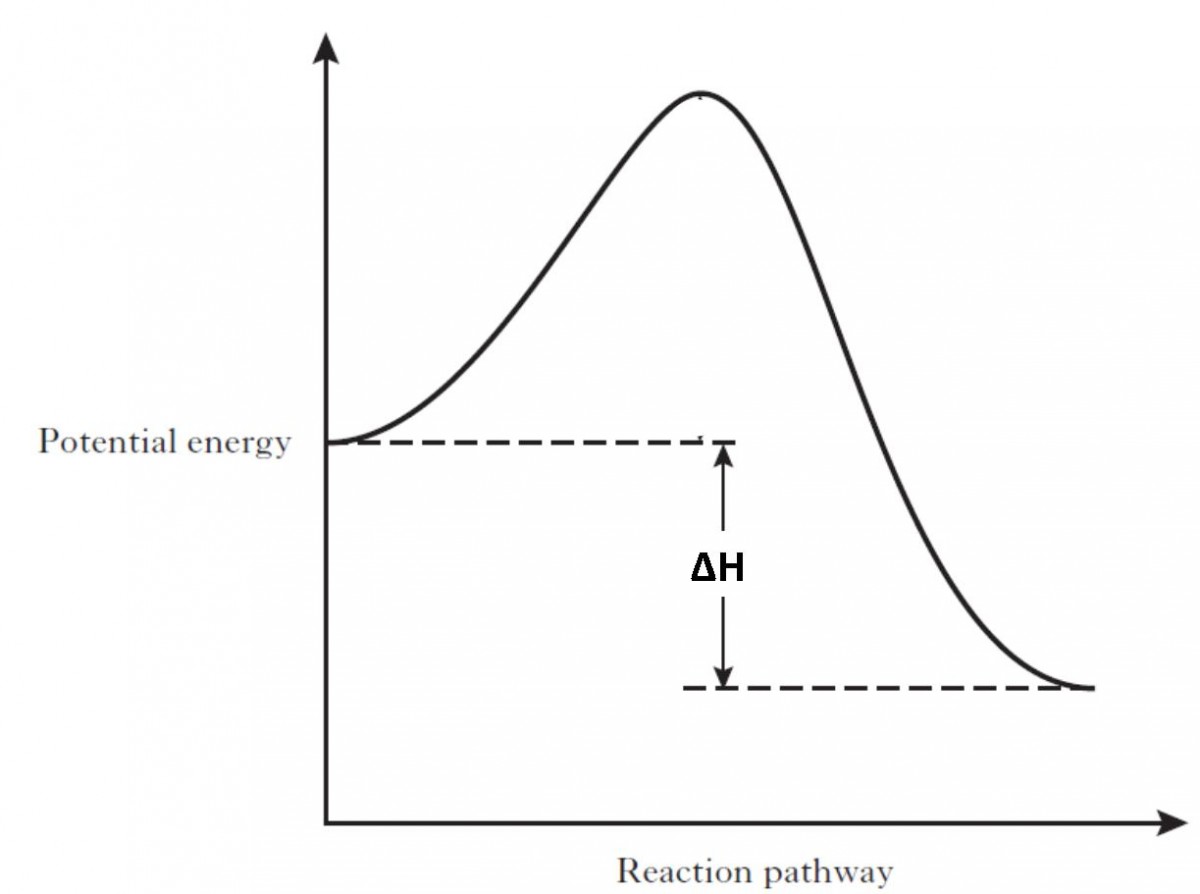
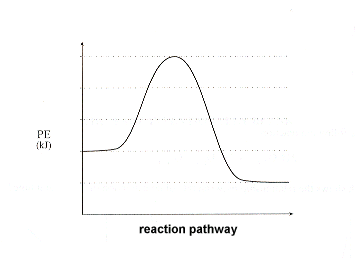
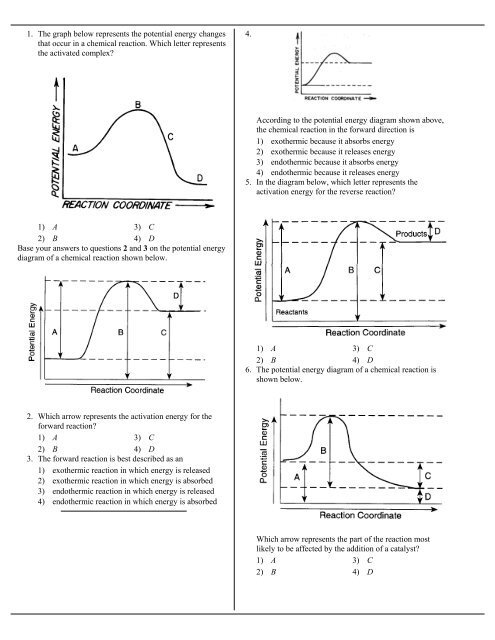
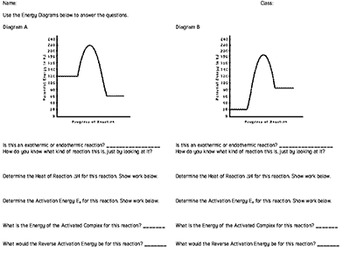
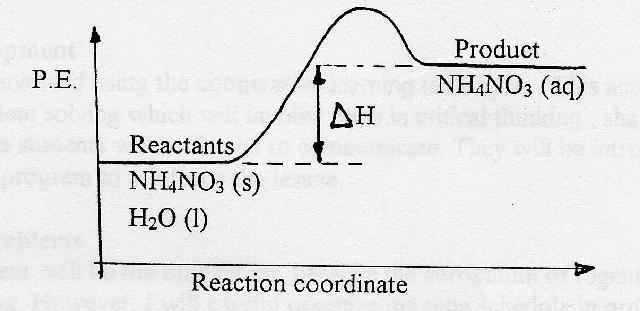
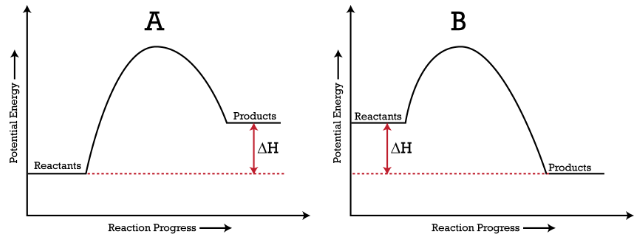
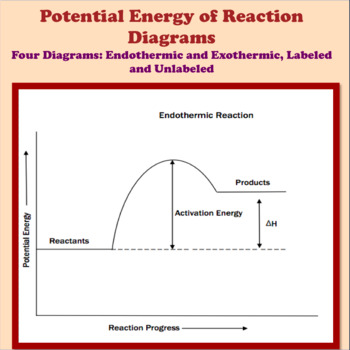
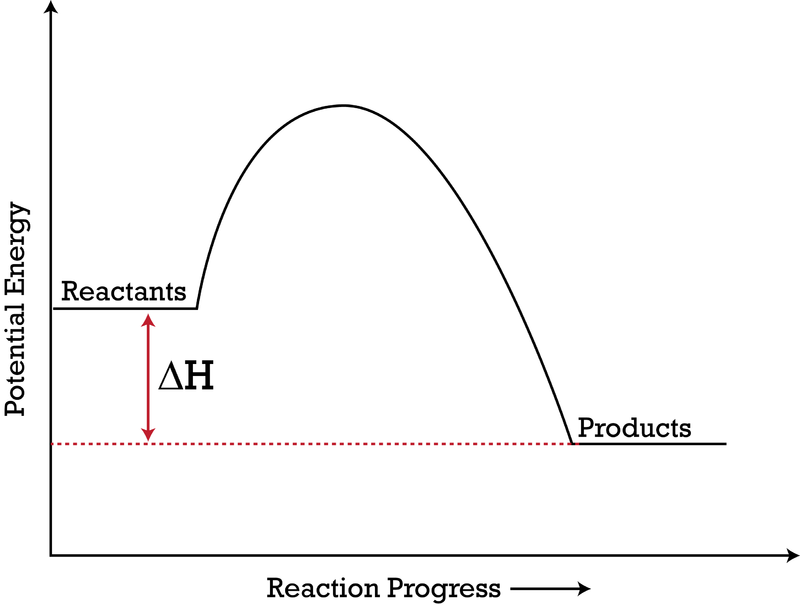
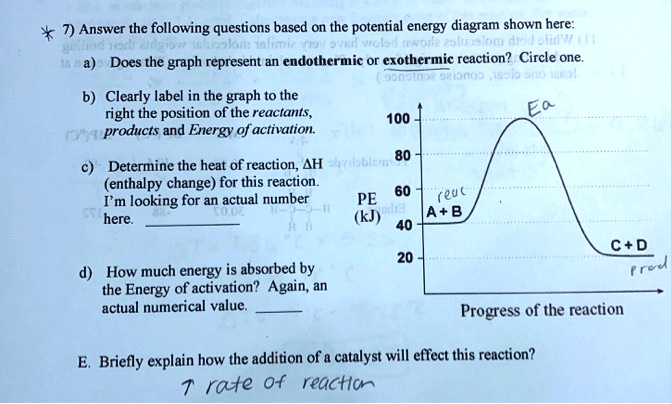
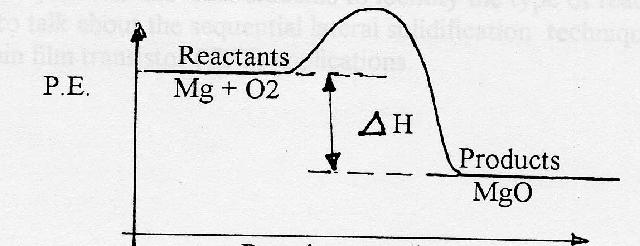
44 potential energy diagram for endothermic reaction
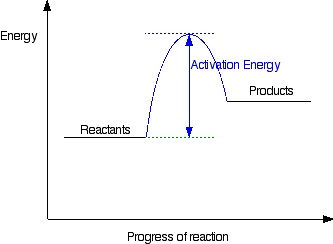
What are Endothermic Reactions? (with Examples & Video) - BYJUS Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ... PPIC Statewide Survey: Californians and Their Government Oct 27, 2022 · Key Findings. California voters have now received their mail ballots, and the November 8 general election has entered its final stage. Amid rising prices and economic uncertainty—as well as deep partisan divisions over social and political issues—Californians are processing a great deal of information to help them choose state constitutional officers and state legislators and to make ...
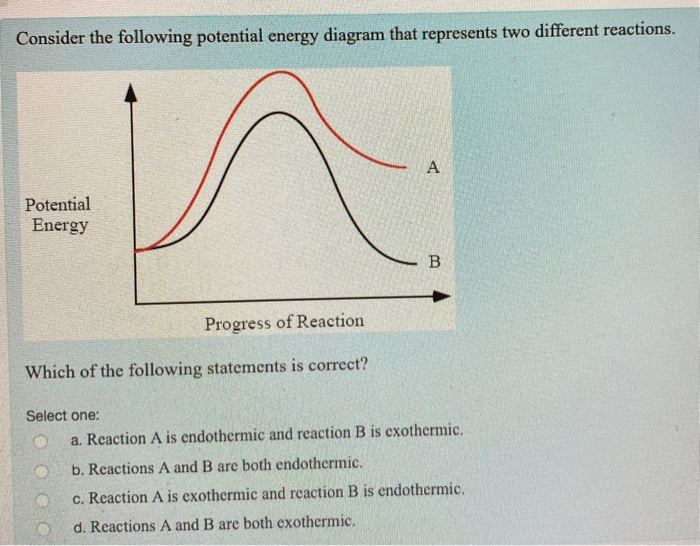
Trends in the Exchange Current for Hydrogen Evolution ... Jan 24, 2005 · The free energy diagrams for the reaction at equilibrium over Pt(111), Ni(111), Mo(110), and Au(111) are shown in Fig. 2. At equilibrium the free energy per H atom (the chemical potential) of the initial and final states of reaction (Eq. 2) are the same. In Fig. 1 and 2 we have also included the free energy of the adsorbed state calculated as

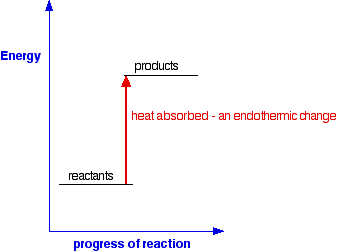
Potential energy diagram for endothermic reaction
Reactions & Rates - Reaction | Kinematics | Concentration ... Explore what makes a reaction happen by colliding atoms and molecules. Design experiments with different reactions, concentrations, and temperatures. When are reactions reversible? Examples of Chemical Energy in Everyday LIfe - YourDictionary Chemical energy is either released (exothermic reaction) or absorbed (endothermic reaction) during a chemical reaction. In an exothermic reaction, heat is released, creating warmth. In an endothermic reaction, the heat is absorbed, creating cooling. Air bags are activated by a chemical reaction inside the bag. A sensor turns on an electrical ... Energy - Wikipedia The total energy of a system can be subdivided and classified into potential energy, kinetic energy, or combinations of the two in various ways. Kinetic energy is determined by the movement of an object – or the composite motion of the components of an object – and potential energy reflects the potential of an object to have motion, and generally is a function of the position of an object ...
Potential energy diagram for endothermic reaction. Over 56.55% Faradaic efficiency of ambient ammonia synthesis ... Jan 21, 2019 · Even if *H is adsorbed, its desorption to form H 2 is still an endothermic reaction, with a much stronger Gibbs free energy change than those of common transition and noble metals 24, as shown in ... Energy - Wikipedia The total energy of a system can be subdivided and classified into potential energy, kinetic energy, or combinations of the two in various ways. Kinetic energy is determined by the movement of an object – or the composite motion of the components of an object – and potential energy reflects the potential of an object to have motion, and generally is a function of the position of an object ... Examples of Chemical Energy in Everyday LIfe - YourDictionary Chemical energy is either released (exothermic reaction) or absorbed (endothermic reaction) during a chemical reaction. In an exothermic reaction, heat is released, creating warmth. In an endothermic reaction, the heat is absorbed, creating cooling. Air bags are activated by a chemical reaction inside the bag. A sensor turns on an electrical ... Reactions & Rates - Reaction | Kinematics | Concentration ... Explore what makes a reaction happen by colliding atoms and molecules. Design experiments with different reactions, concentrations, and temperatures. When are reactions reversible?






















0 Response to "44 potential energy diagram for endothermic reaction"
Post a Comment