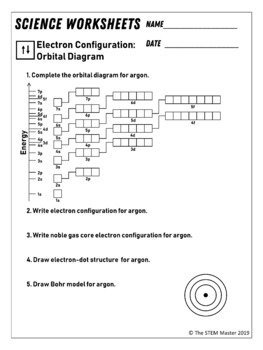
41 orbital diagram of argon
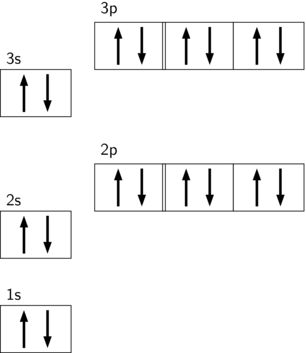
orbital diagram for argon Beryllium diagram element representation ... If you are searching about Show The Orbital Filling Diagram For Br Bromine you've visit to the right place. We have 9 Pictures about Show The Orbital Filling Diagram For Br Bromine like Argon, atomic structure - Stock Image C018/3699 - Science Photo Library, Electron Configuration And Orbital Diagram For Aluminum - Diagram Media and also Science Presentation by Luis Miguel Bulosan. Argon Orbital diagram, Electron configuration, and Valence electrons The first shell of Argon has 2 electrons and the outer shell or valence shell of Argon has 8 electrons, hence, the number of valence electrons in the Argon atom is 8. The orbital diagram for Argon is drawn by following three principles - the Aufbau principle, Hund's principle, and Pauli's exclusion principle.
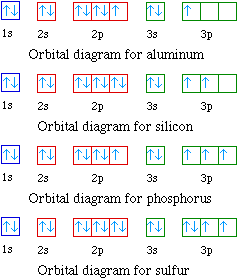
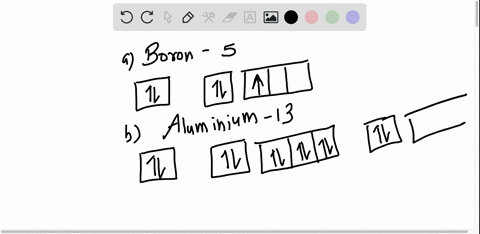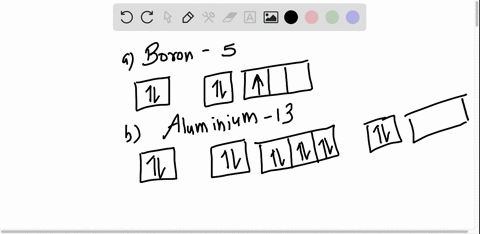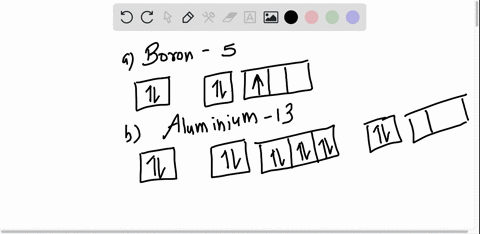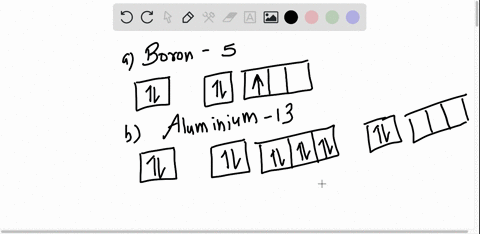
Boron Orbital diagram, Electron configuration, and Valence ... The orbital diagram of Boron contains 1s orbital, 2s orbital, and 2p orbital. 1s orbital contains 1 box, 2s orbital also contains 1 box and 2p orbital contains 3 boxes. Boron has a total of 5 electrons and one box can hold up to the two electrons.

Orbital diagram of argon
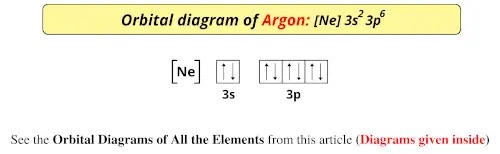
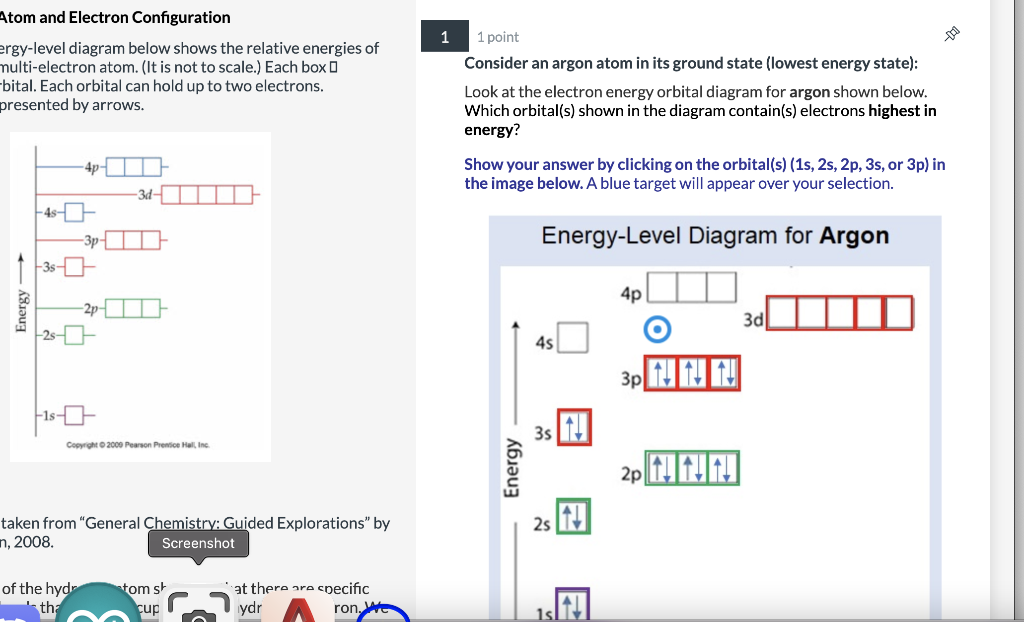
Electron configuration for Argon (element 18). Orbital diagram Density: 0.00166 g/cm 3 . Electronic configuration of the Argon atom: 1s 2 2s 2 2p 6 3s 2 3p 6. Reduced electronic configuration Ar: [Ne] 3s 2 3p 6. Below is the electronic diagram of the Argon atom Distribution of electrons over energy levels in the Ar atom. 1-st level (K): 2. 2-st level (L): 8. 3-st level (M): 8. How to Write the Atomic Orbital Diagram for Argon (Ar) To write the orbital diagram for the Argon (Ar) first we need to write the electron configuration for just Ar. To do that we need to find the number of elec... Orbital diagram - How to draw, Examples, Rules, Filling order - Topblogtenz The orbital diagram will be filled in the same order as described by the Aufbau principle. The order in which the orbitals are filled with electrons from lower energy to higher energy is -. 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p and so on. The above order means -.
Orbital diagram of argon. Argon Electron Configuration - Learnool Methods. We can write the electron configuration of argon using four different methods: #1 Using aufbau principle. #2 Using periodic table. #3 From its bohr model. #4 From its orbital diagram. Let's break down each method in detail. Rubidium(Rb) electron configuration and orbital diagram The 3d orbital is now full. so, the next six electrons will enter the 4p orbital just like the 3p orbital. The 4p orbital is now full. So the remaining one electron will enter the 5s orbital in the clockwise direction. This is clearly shown in the figure of the orbital diagram of rubidium. Rubidium ion(Rb +) electron configuration Argon Orbital diagram, Electron configuration, and Valence For example, the 1s orbital will be filled first with electrons before the 2s orbital. Simply understand that there are commonly four different types of subshells - s, p, d, and, f. These subshells can hold a maximum number of electrons on the basis of a formula, 2(2l + 1) where 'l' is the azimuthal quantum number. Orbital Diagram of Argon - YouTube This video shows how to create an orbital diagram of an atom from its electronic configuration
Write the complete orbital configuration for argon. Using atomic orbital notation (e.g. 1s2 2s2 2p6 ...), write out the complete electronic configuration for Fr. Identify the valence electrons. Give the electron configuration for zirconium and argon. Write the full electron configuration of As. Write the condensed electron configuration for tellurium and state the number of valence electrons. orbital diagram for argon - LoreneBrodee To write the orbital diagram of magnesiumMg you have to do the electron configuration of magnesium. Energy diagram demonstrating the energetics of the photoelectron effect. Of the four substances shown water ammonia carbon dioxide and argon water would freeze first when temperatures fall and the last to sublimate when temperatures rise. Argon Orbital Diagram - Learnool #3 Draw Orbital Diagram of Argon. Before drawing the orbital diagram, you should know the three general rules. Aufbau principle - electrons are first filled in lowest energy orbital and then in higher energy orbital; Pauli exclusion principle - two electrons with the same spin can not occupy the same orbital; 13+ orbital diagram for argon - NorteyLalana The first shell of Argon has 2 electrons and the outer shell or valence shell of Argon has 8 electrons hence the number of valence electrons in the Argon atom is 8. 3 From its bohr model. The number of electrons in the atom is. Heres how you can draw the orbital diagram of argon step by step. 3 Draw Orbital Diagram of Argon. 1 Using aufbau ...
Potassium(K) electron configuration and orbital diagram Then the next two electrons will enter the 3s orbital just like the 1s orbital and the next six electrons will enter the 3p orbital just like the 2p orbital. The 3p orbital is now full. So, the remaining one electron will enter the 4s orbital in the clockwise direction. This is clearly shown in the figure of the orbital diagram of potassium. Orbital Diagram of all Elements (118 Orbital Diagrams Inside) Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... Argon (Ar) 19: Potassium (K) 20: Calcium (Ca) 21: Scandium (Sc) 22: Titanium (Ti) 23: Vanadium (V) 24: Chromium (Cr) 25: Manganese (Mn) 26: Iron (Fe ... Magnesium Orbital diagram, Electron configuration, and ... What is the orbital diagram for Magnesium (Mg)? The orbital diagram for Magnesium is drawn with 4 orbitals. The orbitals are 1s, 2s, 2p, and 3s. The Magnesium orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, and the remaining two electrons in the 3s orbital.
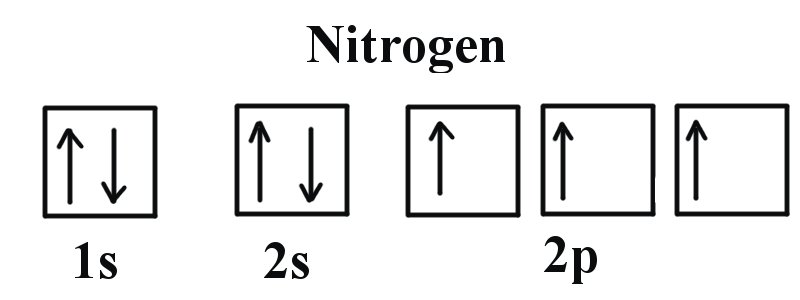
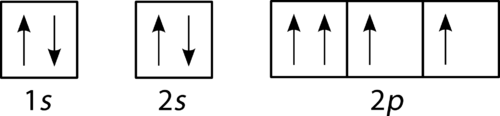
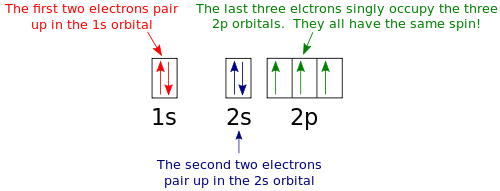
Carbon(C) electron configuration and orbital diagram Orbital Diagram for Carbon Electron configuration of carbon in the excited state. Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. We already know that the p-subshell has three orbitals.
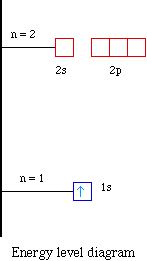
Argon(Ar) electron configuration and orbital diagram And Pauli’s exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. To write the orbital diagram of argon(Ar), you have to do the electron configuration of argon. Which has been discussed in detail above. 1s is the closest and lowest energy orbital to the nucleus.
Atomic orbital - Wikipedia Atomic orbitals can be the hydrogen-like "orbitals" which are exact solutions to the Schrödinger equation for a hydrogen-like "atom" (i.e., atom with one electron). ). Alternatively, atomic orbitals refer to functions that depend on the coordinates of one electron (i.e., orbitals) but are used as starting points for approximating wave functions that depend on the simultaneous coordinates of ...
Electron Configuration for Argon (Ar) - UMD When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. The next six electrons will go in the 2p orbital.
Give the symbol of the atom with the orbital diagram beyond argon. Okay, so for this first one, we'll go from our orbital diagram to an electron configuration, they said it's after Oregon. So for us to three D eight. So you can either count over on the periodic table and see that that's nickel Or you can see that you have 18 close to of course a is 28 electrons which matches up with nickel. Okay, the next one we've got are gone for us to three D five.
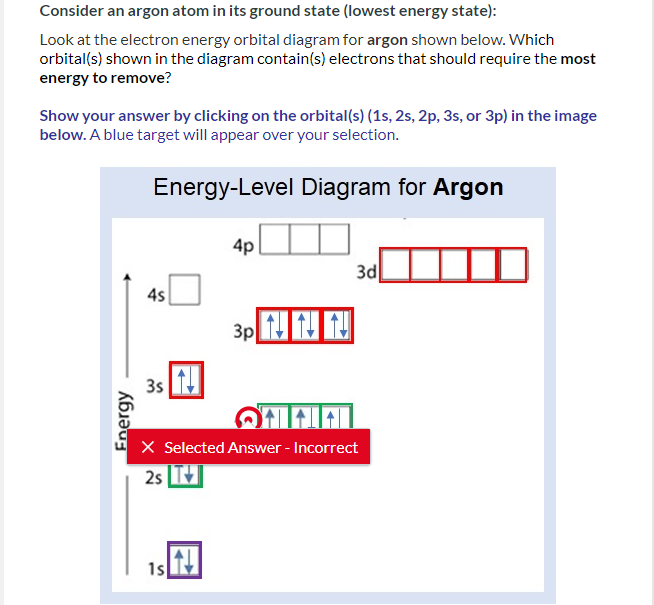
Solved QUESTION 20 When completing the orbital diagram for | Chegg.com Science. Chemistry. Chemistry questions and answers. QUESTION 20 When completing the orbital diagram for the element argon, which of the following statements is correct? The 3p sublevel is not full. There are no electrons in the 4s sublevel. There are electrons in the 3d sublevel. There is one unpaired electron in the 3p sublevel. There are six ...
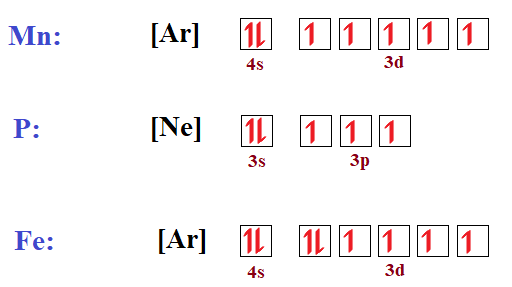
How to Do Orbital Diagrams | Sciencing This works by using the noble gases (in the far right column of the periodic table) as a starting point and adding the final orbitals onto them. So scandium has the same configuration as argon, except with electrons in two extra orbitals. The shorthand form is therefore: [Ar] 4s 2 3d 1.
Orbital diagram - How to draw, Examples, Rules, Filling order - Topblogtenz The orbital diagram will be filled in the same order as described by the Aufbau principle. The order in which the orbitals are filled with electrons from lower energy to higher energy is -. 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p and so on. The above order means -.
How to Write the Atomic Orbital Diagram for Argon (Ar) To write the orbital diagram for the Argon (Ar) first we need to write the electron configuration for just Ar. To do that we need to find the number of elec...
Electron configuration for Argon (element 18). Orbital diagram Density: 0.00166 g/cm 3 . Electronic configuration of the Argon atom: 1s 2 2s 2 2p 6 3s 2 3p 6. Reduced electronic configuration Ar: [Ne] 3s 2 3p 6. Below is the electronic diagram of the Argon atom Distribution of electrons over energy levels in the Ar atom. 1-st level (K): 2. 2-st level (L): 8. 3-st level (M): 8.













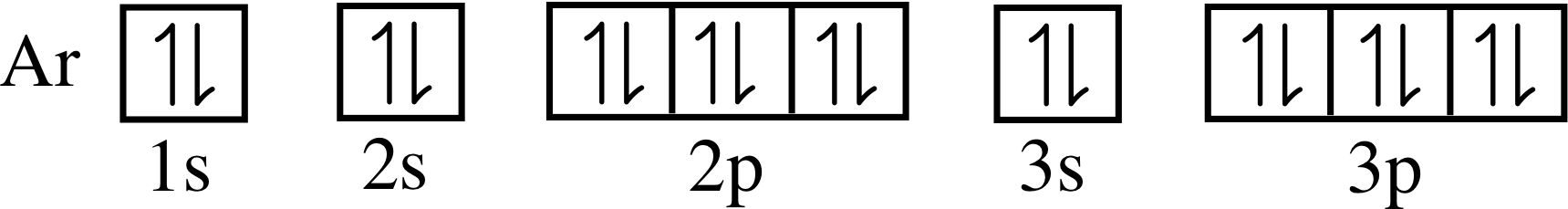








0 Response to "41 orbital diagram of argon"
Post a Comment