41 ni2+ orbital diagram
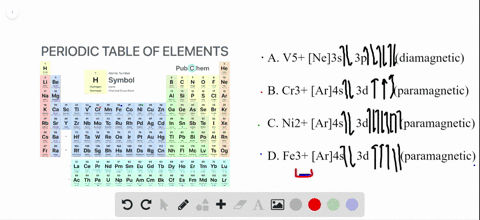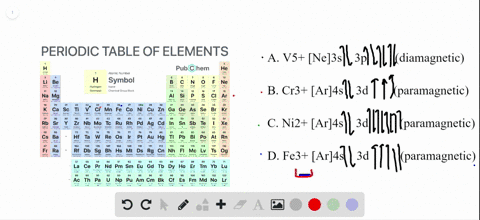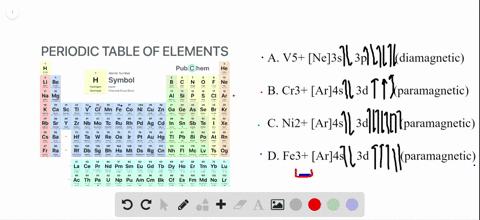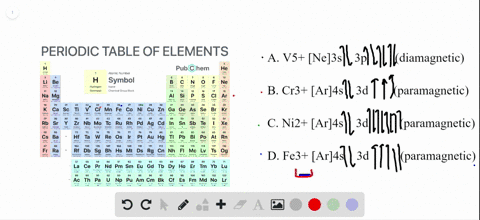
Write orbital diagrams for each of these ions....open 8 Answer of Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ Schematic level diagrams of the Ir4+ 5d and Ni2+ 3d orbitals. The ... View publication. Schematic level diagrams of the Ir4+ 5d and Ni2+ 3d orbitals. The down-spin 3z2 − r2 electron mediates a FM coupling via a ddσ hybridization [ (a) and (b)]. The up-spin xz/yz ...
Giant orbital polarization of Ni2+ in a square planar environment ... Abstract. Finding large orbital polarization in Ni-based oxides has become a topic of paramount interest in recent years due to the prospect of finding superconductivity. In this study, we investigate the electronic structure of single-crystalline samples of Sr 2 CuO 3 and Ni-doped Sr 2 CuO 3 containing Cu/NiO 4 square planar units.

Ni2+ orbital diagram
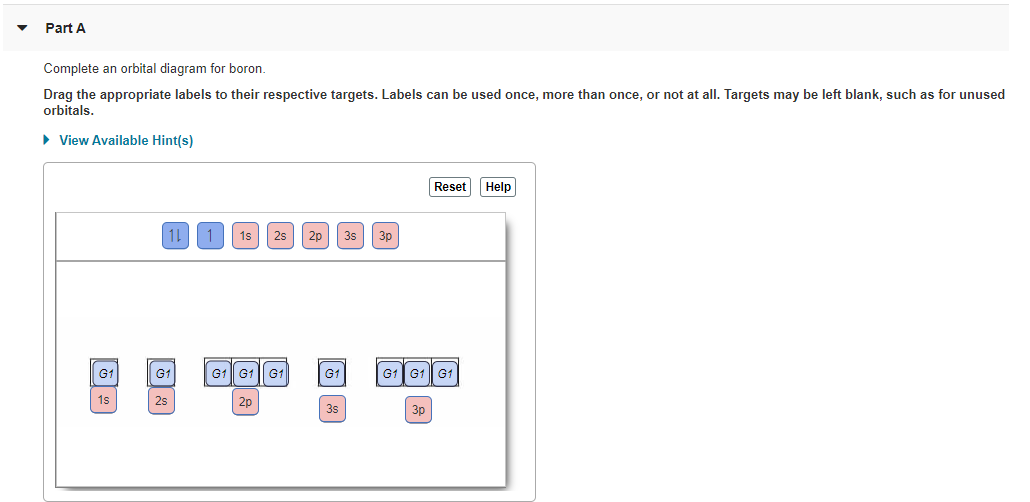
Nickel(Ni) electron configuration and orbital diagram - Valenceelectrons Nickel orbital diagram According to Hunds principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. The 1s orbital is now filled with two electrons. Then the next two electrons will enter the 2s orbital just like the 1s orbital. enter the orbital diagram for the ion cd2+ - JaydeIheoma Heavy Metal Cd2 Ni2 Pb2 And Ni2 Adsorption In Aqueous Solutions By Oxidized Starches Soto 2015 Polymers For Advanced Technologies Wiley Online Library ... Write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. Thus the ground state electron configuration of this element is. Molecular Orbitals for N2 - Newcastle University The orbitals models are shown in two popup windows, which are reused alternately so that you can compare one orbital with another Contours on a two-dimensional plot correspond to surfaces in three dimensions The initial view of a model is with surfaces at ψ = ±0.04 A radio button is provided to 'Switch contours on'.
Ni2+ orbital diagram. Neon(Ne) electron configuration and orbital diagram The first two electrons of neon enter the 1s orbital and the next two electrons enter the 2s orbital. The s-orbital can have a maximum of two electrons. So, the remaining six electrons enter the 2p orbital. Therefore, the neon full electron configuration will be 1s 2 2s 2 2p 6. Note: The short electron configuration of neon is [ He] 2s 2 2p 6. d-orbital for Ni2+ in the Ni(II) semisepulchrates - ScienceDirect A d -orbital for the Ni 2+ ion in the nickel (II) semisepulchrates is proposed by considering the ligand charge penetration and using a simple point-charge model. The d-d transition energies for Ni 2+ in the complexes are calculated using the corresponding structural data. The theoretical results are in good agreement with experimental findings. Solved Write orbital diagrams for each of these | Chegg.com Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ Expert Answer 84% (44 ratings) Elementary Vanadium has electron configuration [Ar] 4s2 3d3 . Hence V5+⁵ions have the same electron configuration as argon: [V5+] = [Ar] = 1s2 2s2 2 … View the full answer SOLVED: Draw a d-orbital splitting diagram for Ni2+ in a ... - Numerade VIDEO ANSWER:first compound given is Kathie Ian old tries. Okay plus two here, iron contained plus two charts and its outermost electronic configuration of thi…
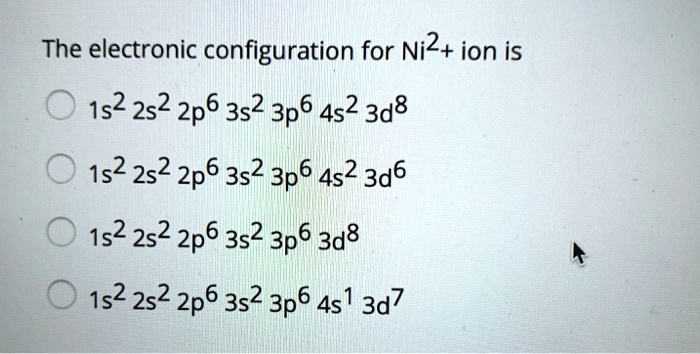
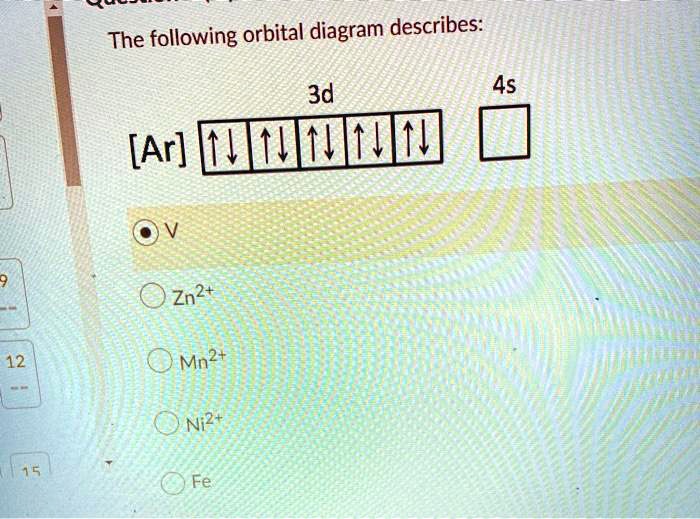
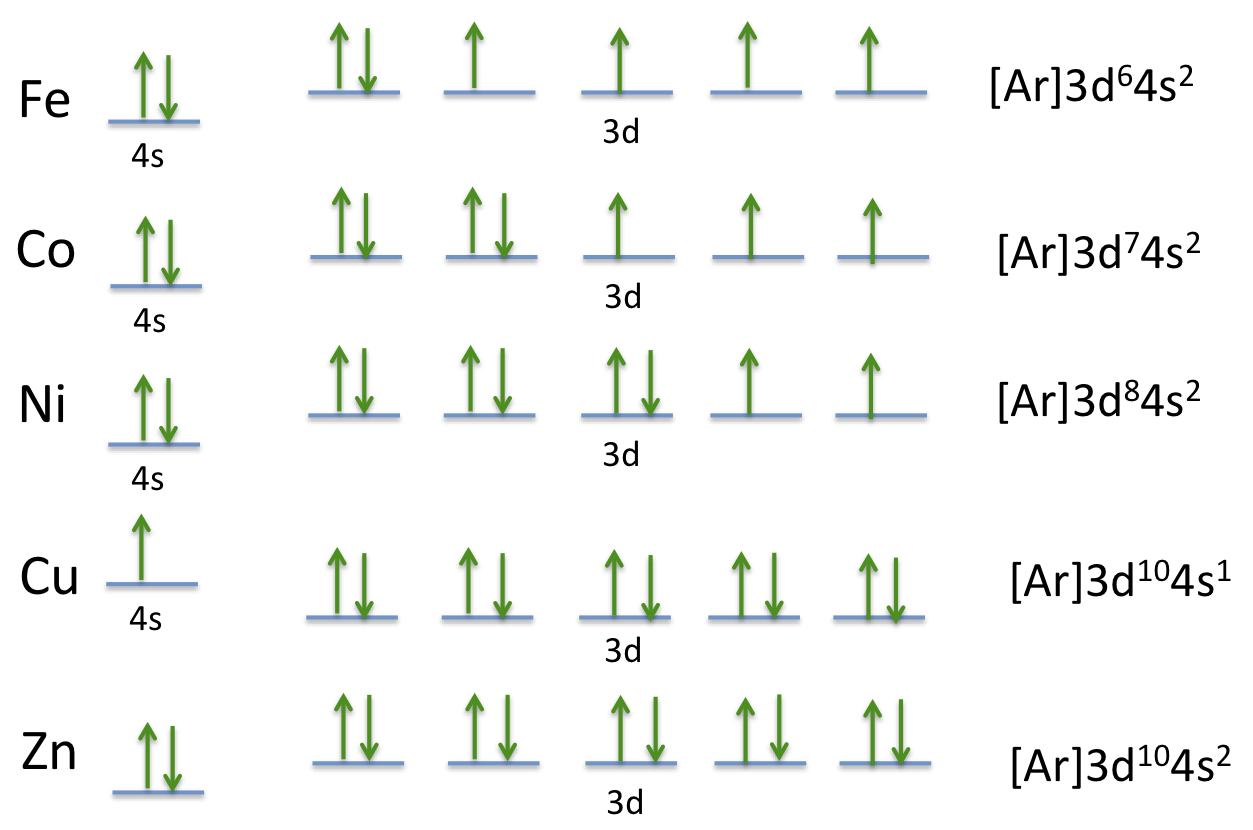
Nitrogen Orbital diagram, Electron configuration, and ... - Topblogtenz The first shell of nitrogen has 2 electrons and the outer shell or valence shell of nitrogen has 5 electrons, hence, the number of valence electrons in the nitrogen atom is 5. The orbital diagram for nitrogen is drawn by following three principles - the Aufbau principle, Hund's principle, and Pauli's exclusion principle. What is the molecular orbital diagram for NO₂? - Quora It's the sequence of the energy levels in the molecular orbital scheme for n-2 diatomics. after that, just count the number of electrons and put them into the energy levels according to Aufbau and Hund. Let's count the valence electrons. B has 3, F has 7 but the + charge means remove one of those electrons. So, 3+7-1 = 9. Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3 ... The complex [Zn(NH3)4] 2+ is diamagnetic, but [Ni(NH3)4] 2+ is not. Explain this difference by means of orbital energy diagrams. Determine the spectroscopic ground state for the Ni2+ ion. What spectral transitions would an octahedral complex of this... What is the electron configuration of Ni^(2+)? | Socratic Explanation: Electronic configuration of Nickel ( 28Ni) is 1s2 2s2 2p6 3s2 3p6 4s2 3d8 After removal of two electrons from outermost shell the electron configuration of Ni2+ is 1s2 2s2 2p6 3s2 3p6 4s0 3d8 Answer link
Molecular Orbital Diagram For Ne2 theory, we will formalize a definition of bond order--the number of bonds between atoms in a molecule. molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Orbital filling diagrams | The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... MO Diagram for N2+ (Molecular Orbital) - YouTube MO Diagram for N2+ (Molecular Orbital) 497,470 views Feb 27, 2013 3K Dislike Share Save chemistNATE 223K subscribers There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2,... Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration Here we will get you the information with the valence electrons that nitrogen has. There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Apart from that one more thing is unique about the element, i.e, nitrogen can have either one of 3 or 5 valence electrons.
Answered: Write orbital diagrams for each ion and… | bartleby V5 + b. Cr3 + c. Ni2 + d. Fe3 + Question. Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. V5 + b. Cr3 + c. Ni2 + d. Fe3 + Expert Solution. ... D. Draw the orbital diagram of each element and determine if the ground state of each element is ...
Nickel - Electron Configuration and Oxidation States - Ni - Periodic Table Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. It is a soft, silvery-white alkali metal. Under standard conditions, it is the lightest metal and the lightest solid element. Like all alkali metals, lithium is highly reactive and flammable, and is stored in mineral oil.
Energy level diagram for Molecular orbitals - Class Notes 2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals.. Bond Order = ½ ( N b - Na). The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero. 3) Relative stability of molecule in terms ...
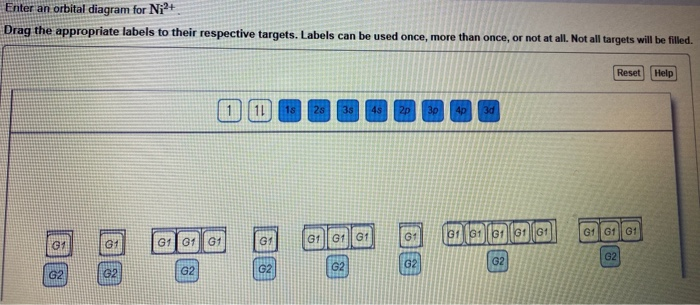
Solved 1. Write the electronic configuration of Ni2+. 2. - Chegg 1. Write the electronic configuration of Ni2+. 2. Draw an orbital energy level diagram for the electrons in Ni2+. Question: 1. Write the electronic configuration of Ni2+. 2. Draw an orbital energy level diagram for the electrons in Ni2+.
Electron Configuration for Ni, Ni2+, and Ni3+ (Nickel and ... - YouTube We first need to find the number of electrons for the Ni atom (there are 28 electrons) using the Periodic Table. When we write the configuration, we'll put all 28 electrons in orbitals around the...
OneClass: For Ni2+, draw an orbital energy diagram and place the ... For Ni2+, draw an orbital energy diagram and place the valence electrons in the diagram that would indicate that it is in an excited state. Transition Metal Chemistry and Paper Chromatography would indicate that it is an excited state, Show full question + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required.
Ni2+ Electron Configuration: 3 Facts You Should Know From the box diagram and Ni2+ electron configuration, it is evident that among 8 electrons of Ni 2+ in d orbital, six electrons are paired in d xy, d yz, d zx, orbitals, and two electrons remain in unpaired form in d x2 - y2 and d z2 orbital. So, the number of unpaired electrons in Ni 2+ is two.
1. Write orbital diagrams for each of these ions. *a. V5+ *b. Cr3+ *c ... Write the abbreviated orbital diagrams for the following elements and state whether they are paramagnetic or diamagnetic. (a) Ni^ {2+} (b) Ca^ {2+} For Ca and P: a. Draw the complete orbital...
What is the orbital diagram for nickel? - Answers The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ...
Molecular Orbitals for N2 - Newcastle University The orbitals models are shown in two popup windows, which are reused alternately so that you can compare one orbital with another Contours on a two-dimensional plot correspond to surfaces in three dimensions The initial view of a model is with surfaces at ψ = ±0.04 A radio button is provided to 'Switch contours on'.
enter the orbital diagram for the ion cd2+ - JaydeIheoma Heavy Metal Cd2 Ni2 Pb2 And Ni2 Adsorption In Aqueous Solutions By Oxidized Starches Soto 2015 Polymers For Advanced Technologies Wiley Online Library ... Write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. Thus the ground state electron configuration of this element is.
Nickel(Ni) electron configuration and orbital diagram - Valenceelectrons Nickel orbital diagram According to Hunds principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. The 1s orbital is now filled with two electrons. Then the next two electrons will enter the 2s orbital just like the 1s orbital.


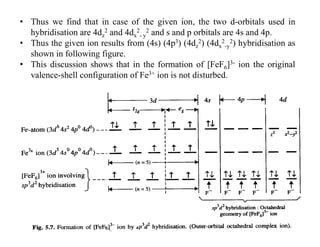







![inorganic chemistry - Why is [PdCl4]2- square planar whereas ...](https://i.stack.imgur.com/xHv3g.png)




0 Response to "41 ni2+ orbital diagram"
Post a Comment