40 orbital filling diagram for nitrogen
p2 molecular orbital diagram - AphraMaiya In the following molecular orbital diagram of N2. Since Nitrogen is a period 2 element we will start the molecular orbital. Web Draw the Molecular Orbital Diagram for P2 P2 P2-. Web We can now fill the molecular orbital diagram. Nitrogen has an electron configuration of 1s 2 2s 2 2p 3. Orbital Diagram of All Elements Diagrams. Electron Configuration for Nitrogen (N) - UMD Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration ...
Solved Show the orbital-filling diagram for N (nitrogen ... - Chegg Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 || 1 18 2s 2p 3s ap Group 1 GT G1 G1 G1 GI GT G161 G2 G2 G2 G2 G2 ; Question: Show the orbital-filling diagram for N (nitrogen ...
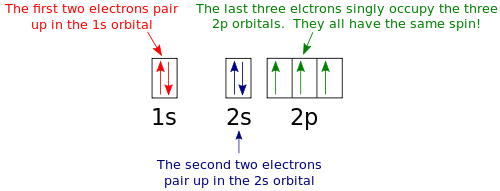
Orbital filling diagram for nitrogen
6.4 Electronic Structure of Atoms (Electron Configurations) For example, after filling the 3p block up to Ar, we see the orbital will be 4s (K, Ca), followed by the 3d orbitals. Figure 6.26 This diagram depicts the energy order for atomic orbitals and is useful for deriving ground-state electron configurations. Solved Show the orbital-filling diagram for N (nitrogen ... - Chegg Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. CHEM 123 Sapling Learning Chapter 11 Flashcards | Quizlet Hybrid orbitals, like atomic orbitals, can only hold two electrons, so one 𝑠𝑝3 hybrid orbital on nitrogen holds the lone pair of electrons and the other three are half‑filled. Each half‑filled 𝑠𝑝3 orbital is then able to overlap with the 𝑠 orbitals of the three hydrogen atoms to produce the three N−H σ bonds in NH3 .
Orbital filling diagram for nitrogen. Orbital Filling Diagram For Bromine Item 1: Part A Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for Br (bromine). 5.17 Hund's Rule and Orbital Filling Diagrams - Quizlet In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal energy level and sublevel. Electrons are indicated by arrows inside the circles. Periodic Trends Flashcards | Quizlet Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p³ Item 2: Part C Show the orbital-filling diagram for S (sulfur). Electron Configuration Orbital Diagram Nitrogen - YouTube To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.
Orbital filling diagrams | The Cavalcade o' Chemistry Feb 23, 2016 · The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: Nitrogen Orbital Diagram - Learnool Here's how you can draw the orbital diagram of nitrogen step by step. Step #1: find electrons of nitrogen. Step #2: write electron configuration of nitrogen. Step #3: draw orbital diagram of nitrogen. Let's break down each step in detail. Orbital diagram Calculator - Get instant answer - Topblogtenz We know that the nitrogen atom has a total of 7 electrons that need to be placed into orbitals, now for calculating its orbital diagram, we need to show its electrons in form of an arrow in different boxes using Aufbau, Hund's, and Pauli's exclusion rule. Nitrogen has a total of 7 electrons and its electron configuration is 1s 2 2s 2 2p 3. part A;Show the orbital-filling diagram for N (nitrogen). Order ... Order subshells by energy, with the... part A;Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. part b;Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at ...
8 - Drawing Molecular Orbital Diagrams — Flux Science To fill the diagram, first, we fill each side of the diagram with the electrons according to nitrogen's electron configuration - [He]2s 2 2p 3. Next, we fill the middle section with the molecular orbital's electron configuration using Hund's Rules, just as we do with atomic orbitals. We fill each shell with two electrons before moving to ... Magnesium (Mg) Orbital diagram, Electron configuration, and … Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.It shows the electrons in numbers, It doesn’t show the details on the spin of electrons like the orbital diagram. Valence electrons:- Valence electrons are the simply outermost electron of an atom situated in an outermost shell surrounding an atomic nucleus. Carbon dioxide - Wikipedia Carbon dioxide (chemical formula CO 2) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas.It is a trace gas in Earth's atmosphere at 417 … Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration Here we will get you the information with the valence electrons that nitrogen has. There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Apart from that one more thing is unique about the element, i.e, nitrogen can have either one of 3 or 5 valence electrons.
2.6: Orbital Filling - Chemistry LibreTexts 2.6: Orbital Filling. MO's are filled from the bottom according to the Aufbau principle and Hund's rule, as we learned for atomic orbitals. Question: what is the quantum mechanical basis of Hund's rule? For O 2 (12 valence electrons), we get the MO energy diagram below. The shapes of the molecular orbitals are shown at the right.
orbital filling diagram worksheet orbital configuration electron diagram worksheet electrons answers solved help workshe valence element total core filling chart need. Orbital Box Diagram Phosphorus schematron.org. orbital diagram phosphorus box ion following configuration electron diagrams 3s which formed 3p build most ll 2s put electrons move
Electronic Orbitals - Chemistry LibreTexts The 2s orbital would be filled before the 2p orbital because orbitals that are lower in energy are filled first. The 2s orbital is lower in energy than the 2p orbital. There are 5 d orbitals in the d subshell. A p orbital can hold 6 electrons. Based off of the given information, n=4 and ℓ=3. Thus, there are 3 angular nodes present.
Orbital Diagrams — Overview & Examples - Expii Steps for Drawing an Orbital Diagram Draw a long vertical arrow that points upward. Label the arrow energy. The arrow shows a qualitative representation of increasing orbital energy. Write out the electron configuration to determine which orbitals are filled. Remember, we can use the periodic table to help us.
Orbital Energy Diagram For Nitrogen - ICASMT Fill in the orbital energy diagram foi the vanadium Fill in the orbital. Figure Molecular Orbital Energy-Level Diagram for H2. has an odd number of valence electrons (5 from nitrogen and 6 from oxygen. With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more ...
Oxygen - Wikipedia Orbital diagram, after Barrett (2002), showing the participating atomic orbitals from each oxygen atom, the molecular orbitals that result from their overlap, and the aufbau filling of the orbitals with the 12 electrons, 6 from each O atom, beginning from the lowest-energy orbitals, and resulting in covalent double-bond character from filled ...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same …
Orbital diagram - How to draw, Examples, Rules, Filling order Let’s take an example of the Nitrogen atom to understand the concept of the making of filling the orbital diagram. How to draw an Orbital diagram for Nitrogen? We know that the nitrogen atom has a total of 7 electrons that need to be placed into orbitals, now for drawing its orbital diagram, we need to show its electrons in form of an arrow in different boxes using Aufbau, Hund’s, and ...
Answered: Show the orbital-filling diagram for N… | bartleby Science Chemistry Q&A Library Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets.
Filling of Electrons in Orbitals - Embibe Exams (d) Nitrogen and Oxygen. These atoms each have seven and eight electrons. After six electrons have been accommodated in the manner described above, a vacant \(2{{\text{p}}_{\text{z}}}\) orbital remains, which will be the seat of the seventh electron with the same spin orientation.
Atmosphere of Earth - Wikipedia The three major constituents of Earth's atmosphere are nitrogen, oxygen, and argon.Water vapor accounts for roughly 0.25% of the atmosphere by mass. The concentration of water vapor (a greenhouse gas) varies significantly from around 10 ppm by mole fraction in the coldest portions of the atmosphere to as much as 5% by mole fraction in hot, humid air masses, and …
Sodium(Na) electron configuration and orbital diagram - Valenceelectrons The atomic number of an element is the number of electrons and protons in that element. That is, the number of electrons and protons in the sodium atom is eleven. The sodium electron configuration is 1s 2 2s 2 2p 6 3s 1. The active atomic mass of the sodium atom is 22.98976928. Sodium is an alkali metal.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
Lewis structure - Wikipedia Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
Nitrogen(N) electron configuration and orbital diagram - Valenceelectrons Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. We already know that the p-subshell has three orbitals.
High School Chemistry/Orbital Configurations - Wikibooks, open books ... The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Notice how all three 2 p electrons in the orbital diagram on the left are in separate orbitals, while two of the three 2 p electrons in the diagram on the right are sharing a single orbital.
Microsoft takes the gloves off as it battles Sony for its Activision ... 12.10.2022 · Microsoft is not pulling its punches with UK regulators. The software giant claims the UK CMA regulator has been listening too much to Sony’s arguments over its Activision Blizzard acquisition.
CN- lewis structure, molecular orbital diagram, and, bond order Clearly, Cyanide (CN) lies in a hetero-nuclear diatomic molecular orbital as it contains two different atoms. Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1.
Nitrogen Orbital diagram, Electron configuration, and ... - Topblogtenz The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows-
Hund's Rule and Orbital Filling Diagrams - Course Hero Figure 1. The 2p sublevel, for the elements boron (Z = 5), carbon (Z = 6), nitrogen (Z = 7), and oxygen (Z = 8). According to Hund's rule, as electrons are added to a set of orbitals of equal energy, one electron enters each orbital before any orbital receives a second electron. Orbital Filling Diagrams
How to Do Orbital Diagrams | Sciencing Orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own line, to represent the spins of the electrons too. Electron Configurations Electron configurations are expressed through a notation that looks like this: 1s 2 2s 2 2p 1.
CHEM 123 Sapling Learning Chapter 11 Flashcards | Quizlet Hybrid orbitals, like atomic orbitals, can only hold two electrons, so one 𝑠𝑝3 hybrid orbital on nitrogen holds the lone pair of electrons and the other three are half‑filled. Each half‑filled 𝑠𝑝3 orbital is then able to overlap with the 𝑠 orbitals of the three hydrogen atoms to produce the three N−H σ bonds in NH3 .
Solved Show the orbital-filling diagram for N (nitrogen ... - Chegg Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets.
6.4 Electronic Structure of Atoms (Electron Configurations) For example, after filling the 3p block up to Ar, we see the orbital will be 4s (K, Ca), followed by the 3d orbitals. Figure 6.26 This diagram depicts the energy order for atomic orbitals and is useful for deriving ground-state electron configurations.

0 Response to "40 orbital filling diagram for nitrogen"
Post a Comment