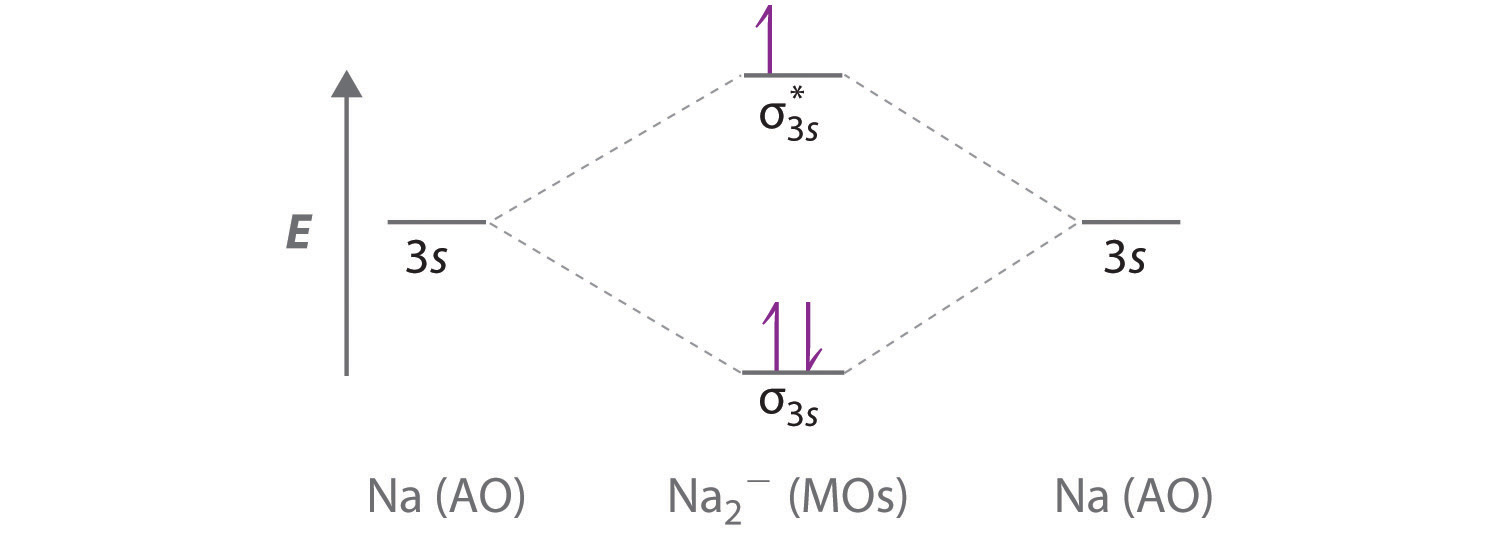
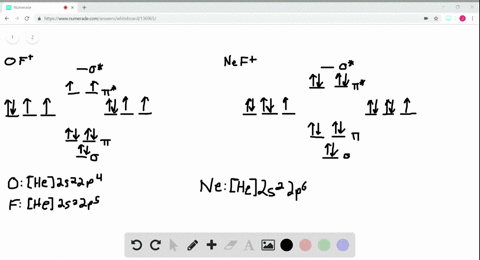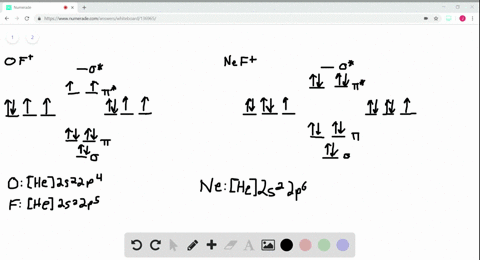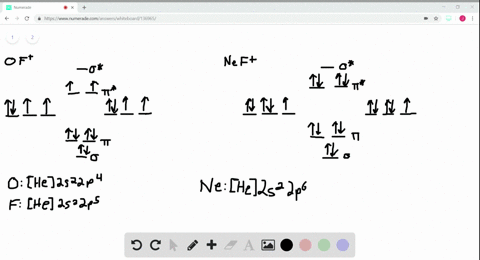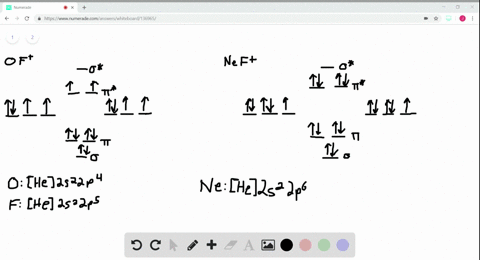
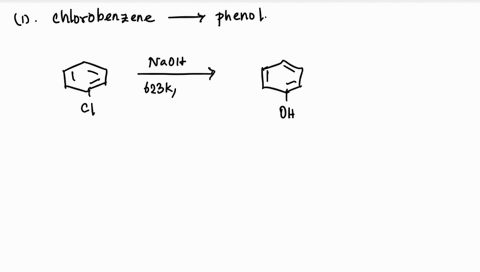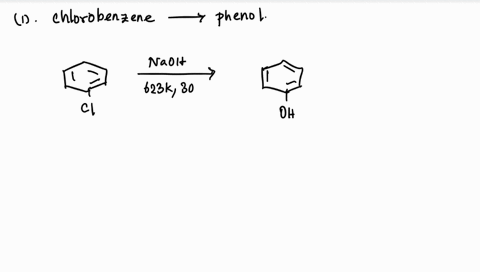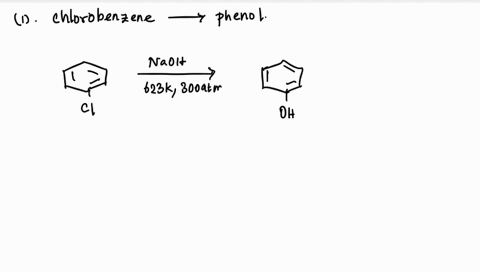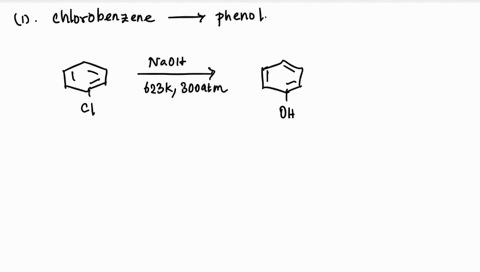
39 use the drawing of the mo energy diagram to predict the bond order of li2+.
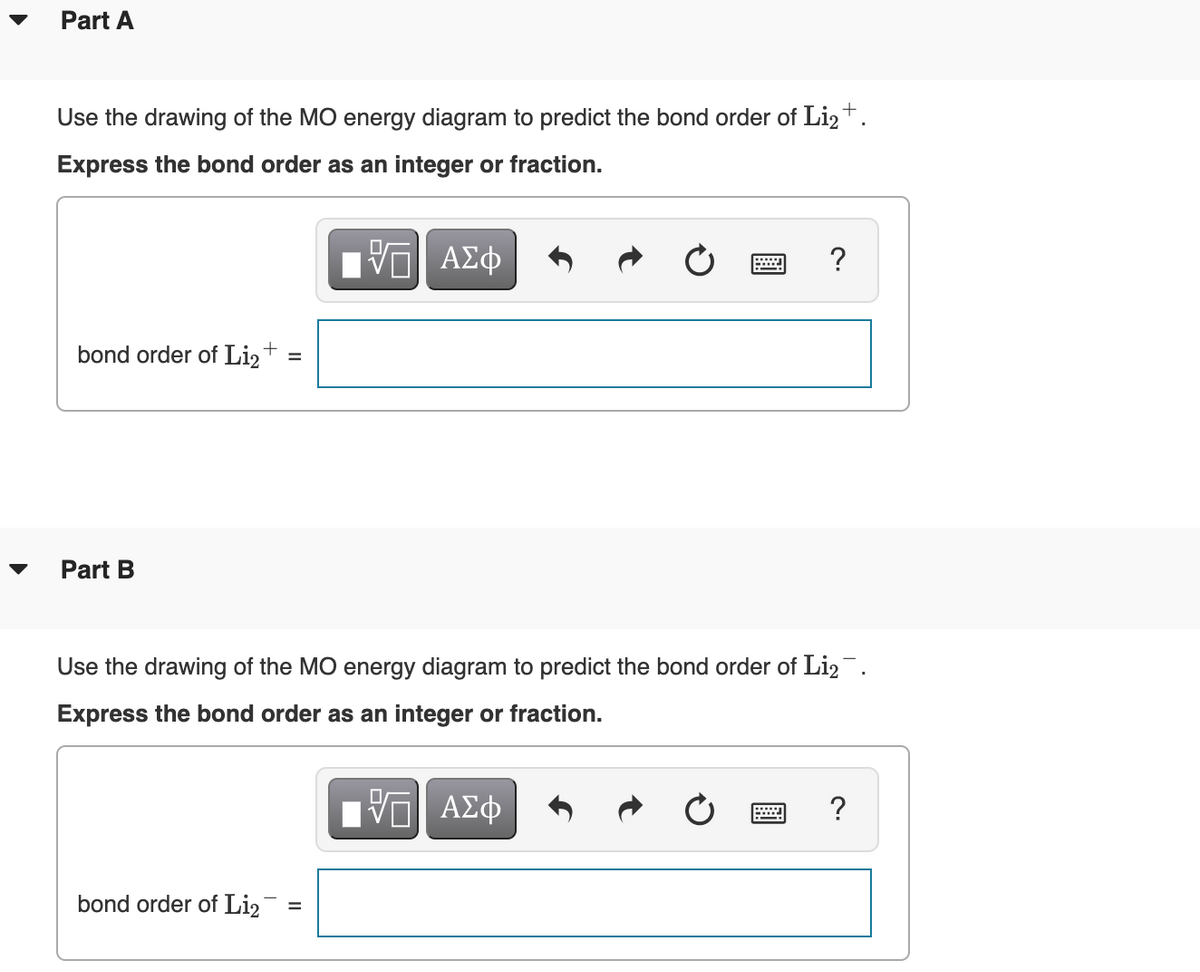
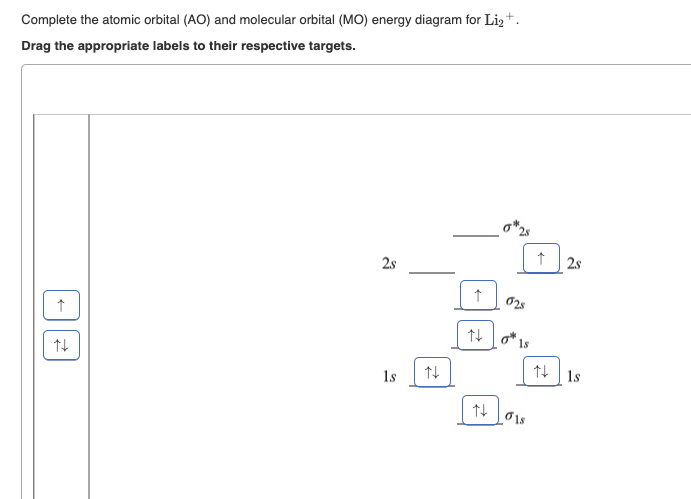
Answered: Draw an MO energy diagram and predict… | bartleby Q: Draw the atomic and molecular orbital (MO) energy diagrams using all the valence electrons for each:…. A: Molecular orbitals are formed by the linear combinations of atomic orbitals. The molecular orbitals…. Q: Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of…. Part A Use the drawing of the MO energy diagram to predict the bond ... Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
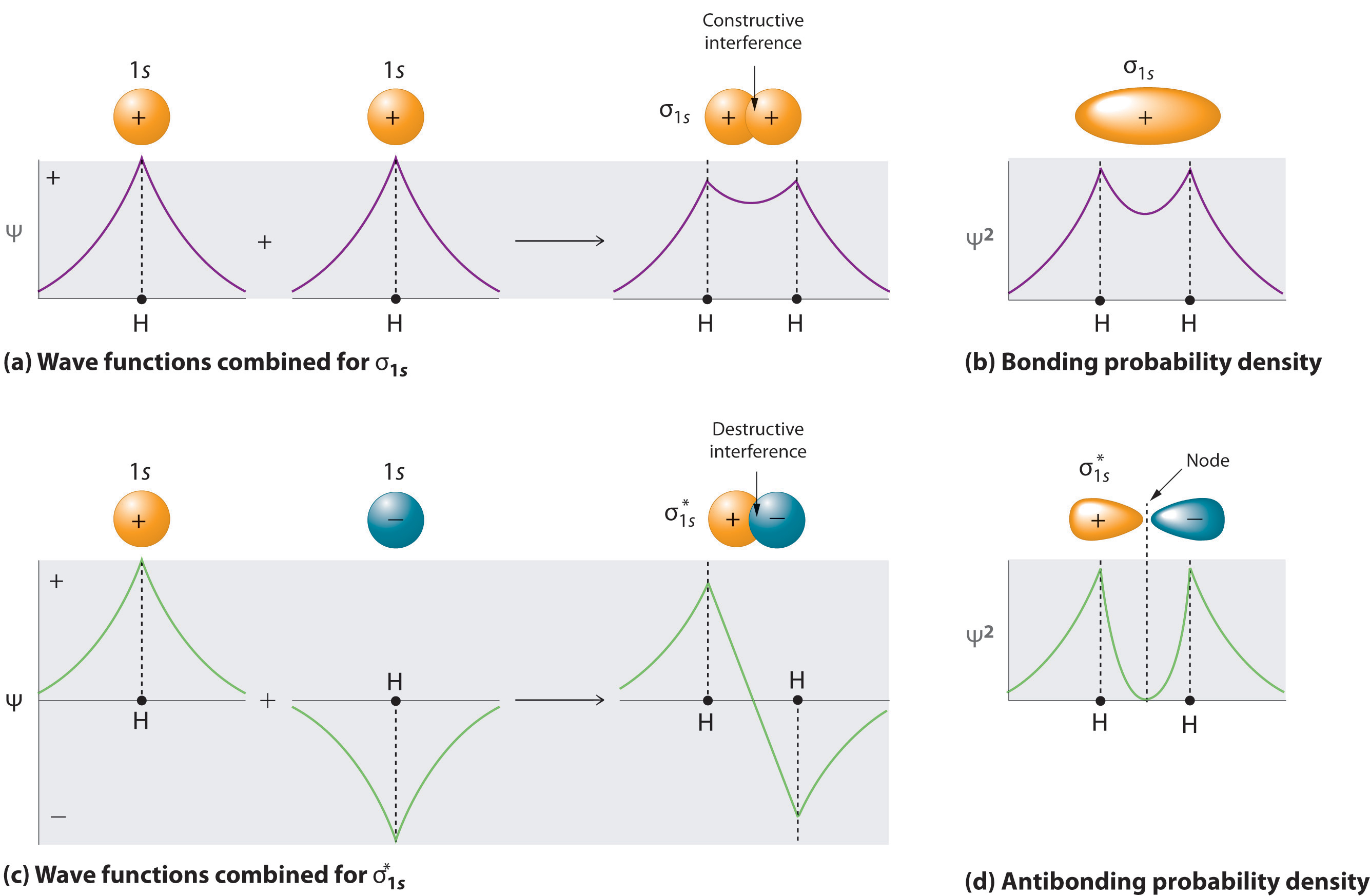
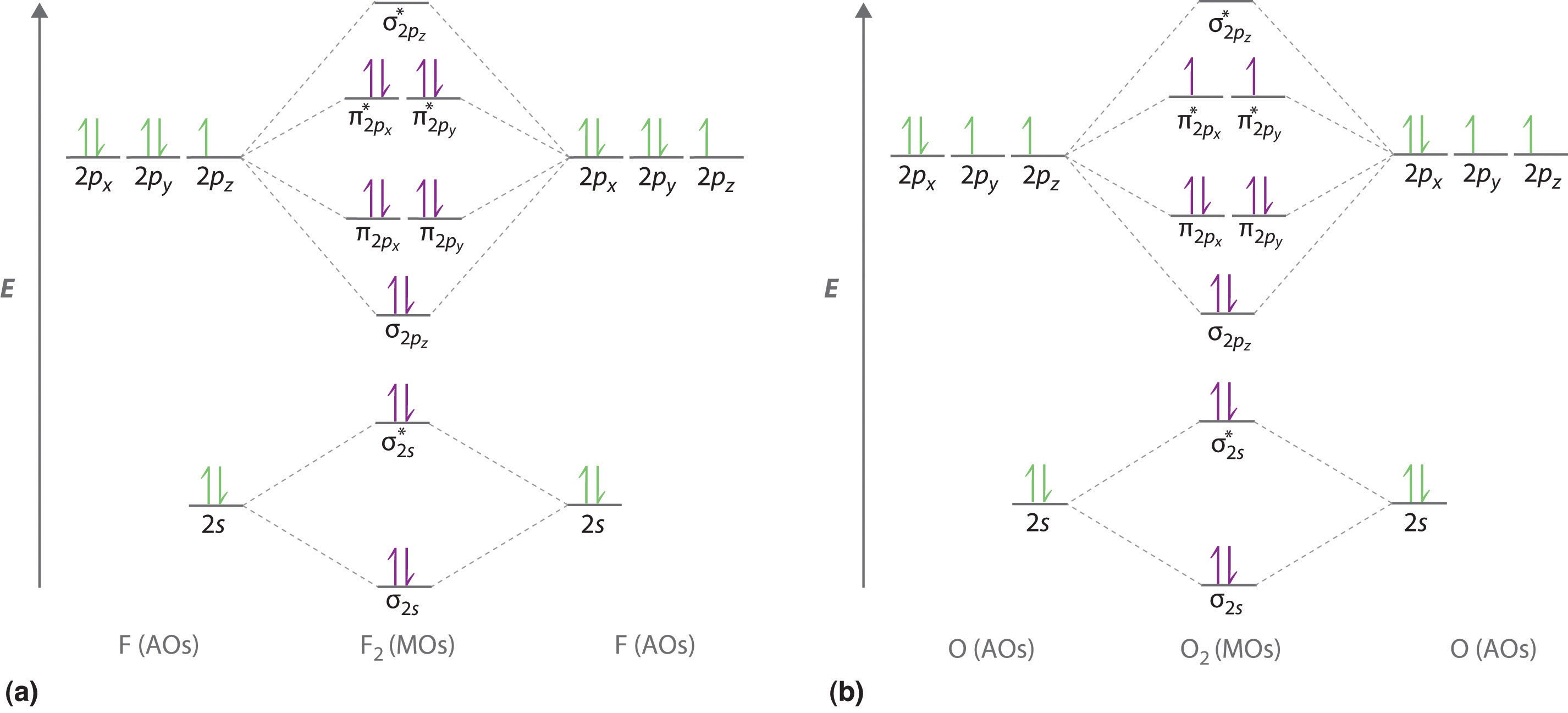
Li2 Molecular Orbital Diagram This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule .

Use the drawing of the mo energy diagram to predict the bond order of li2+.
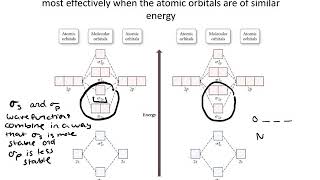
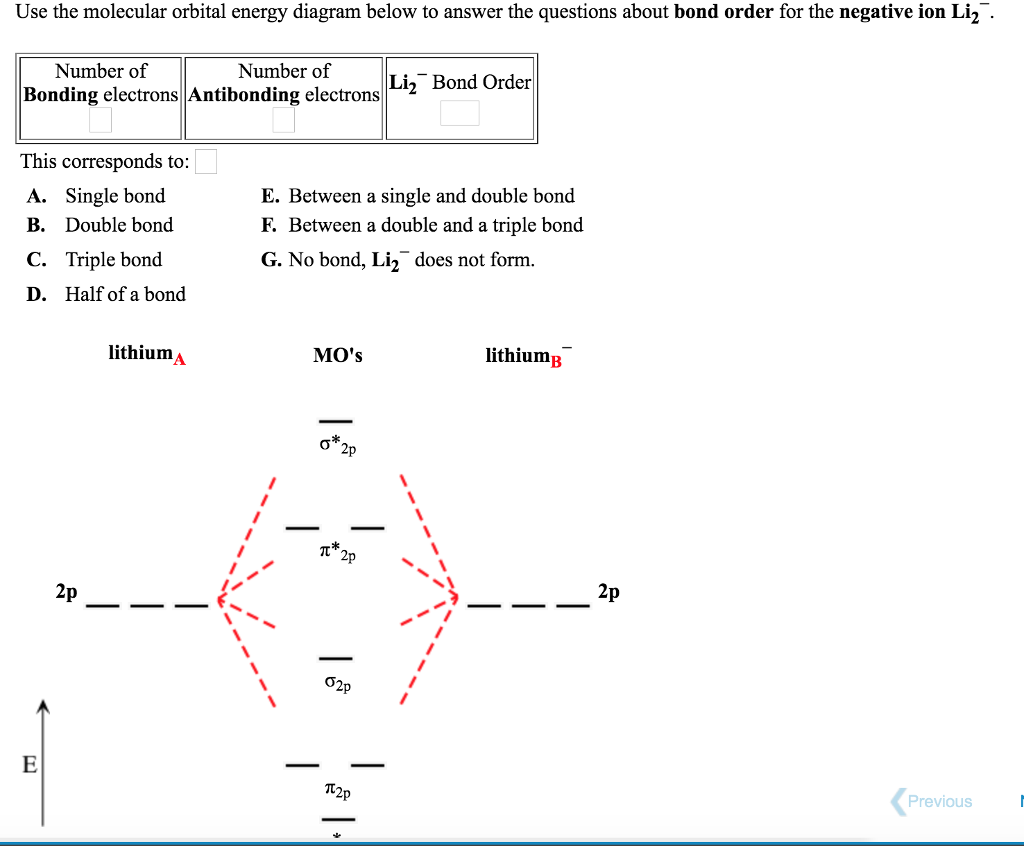
Use the drawing of MO energy diagram for CO to predict the bond order ... Draw an MO energy diagram for HCl. Predict the bond orderand make a sketch of the lowest energy bonding molecularorbital... Draw an MO energy diagram and predict the bond order of Li2 + and Li2 -. Do you expect these molecules to exist in the g... Draw an MO energy diagram and predict the bond order of Be2 + and Be2-. Do you expect these ... Energy level diagram for Molecular orbitals - Class Notes 2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals.. Bond Order = ½ ( N b - Na). The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero. 3) Relative stability of molecule in terms ... How to Make the Molecular Orbital Diagram for Li2- (Bond Order ... The bond order of Li2- is also calculated and the meaning of this numbe... This video discusses how to draw the molecular orbital (MO) diagram for the Li2- ion.
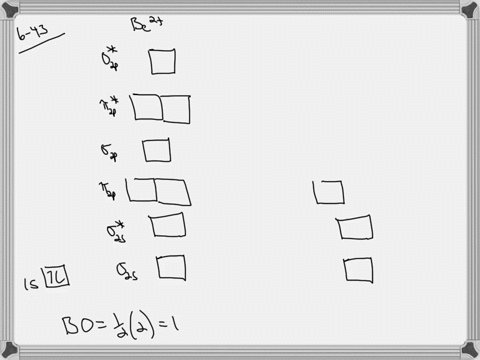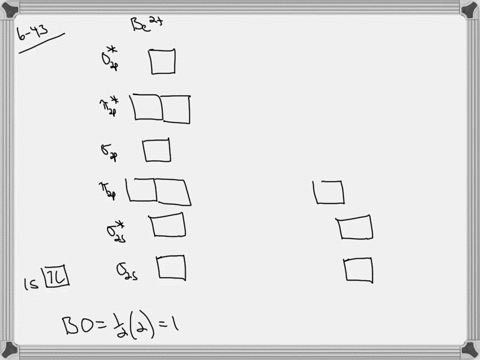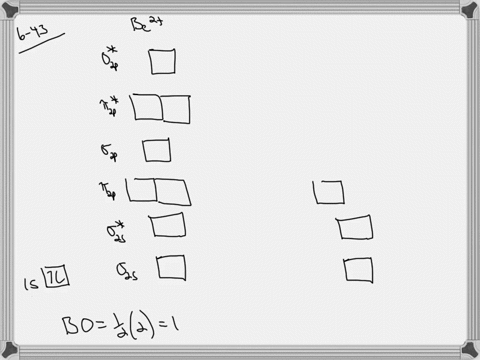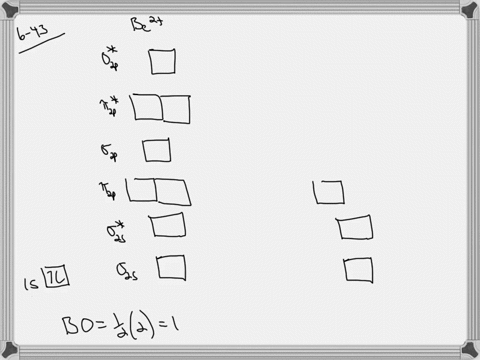
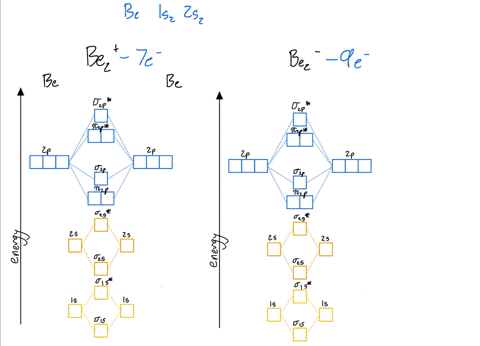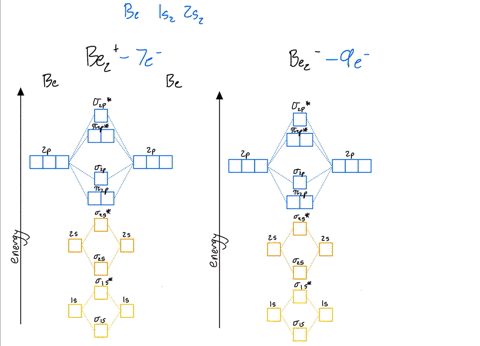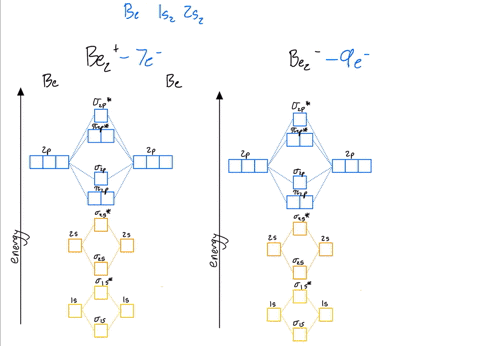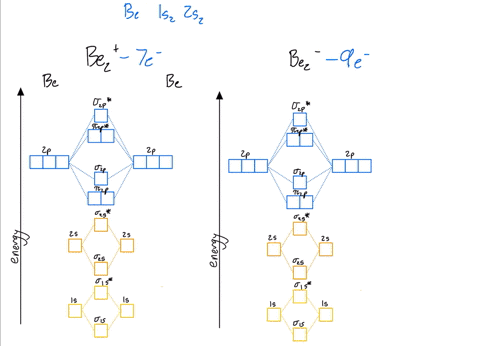
Use the drawing of the mo energy diagram to predict the bond order of li2+.. How does bond order correspond to phase? Use the drawing of MO energy ... How does bond order correspond to phase? Use the drawing of MO energy diagram to predict the bond order of [Be2]+ and [Be2]−. Determined that the bond order of [Be2]+ is (+1/2). Solved Part A Use the drawing of the MO energy diagram to - Chegg Part B Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C Which molecules are predicted to exist in the gas phase? Check all that apply Check; Question: Part A Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an ... mastering chemistry help? Use the drawing of the MO energy diagram to ... Each MO can hold two e⁻s and hence for Li2 the MO scheme is σ(↑↓) σ*(0) Li2 is present the extent of ~1% in Li(g). Removal of one e⁻ from Li2 to give Li2+ results in an MO scheme of σ(↑)σ*(0) Likewise Li2^- has an MO scheme of σ(↑↓) σ*(↑) and also a bond order of 0.5 and again should be observed in the gas phase. For ... Part A Use the drawing of the MO energy diagram to predict the bond ... Q: Draw an MO energy diagram and predict the bond order of Be2 + and Be2 -. Do you expect these molecules to exist in the gas phase? Posted 26 days ago. View Answer . Q: A. In Lewis theory, the two bonds in a double bond look identical. However, valence bond theoryshows that they are not.1.
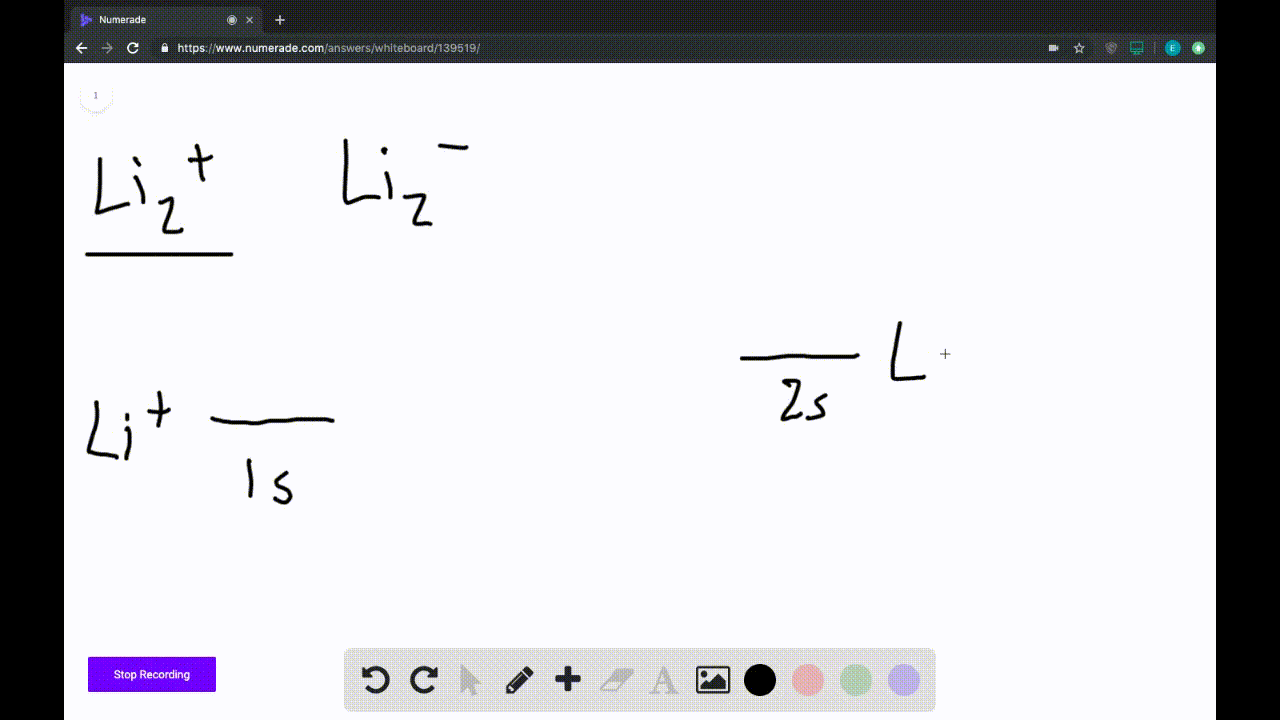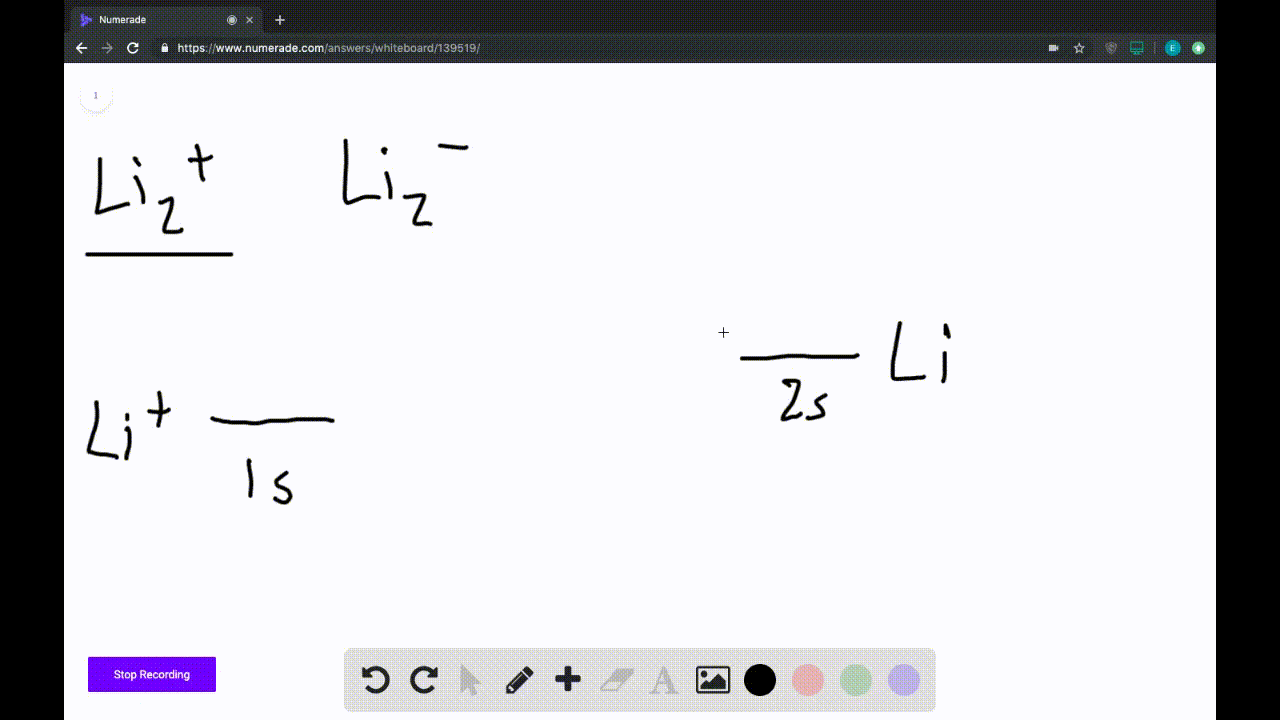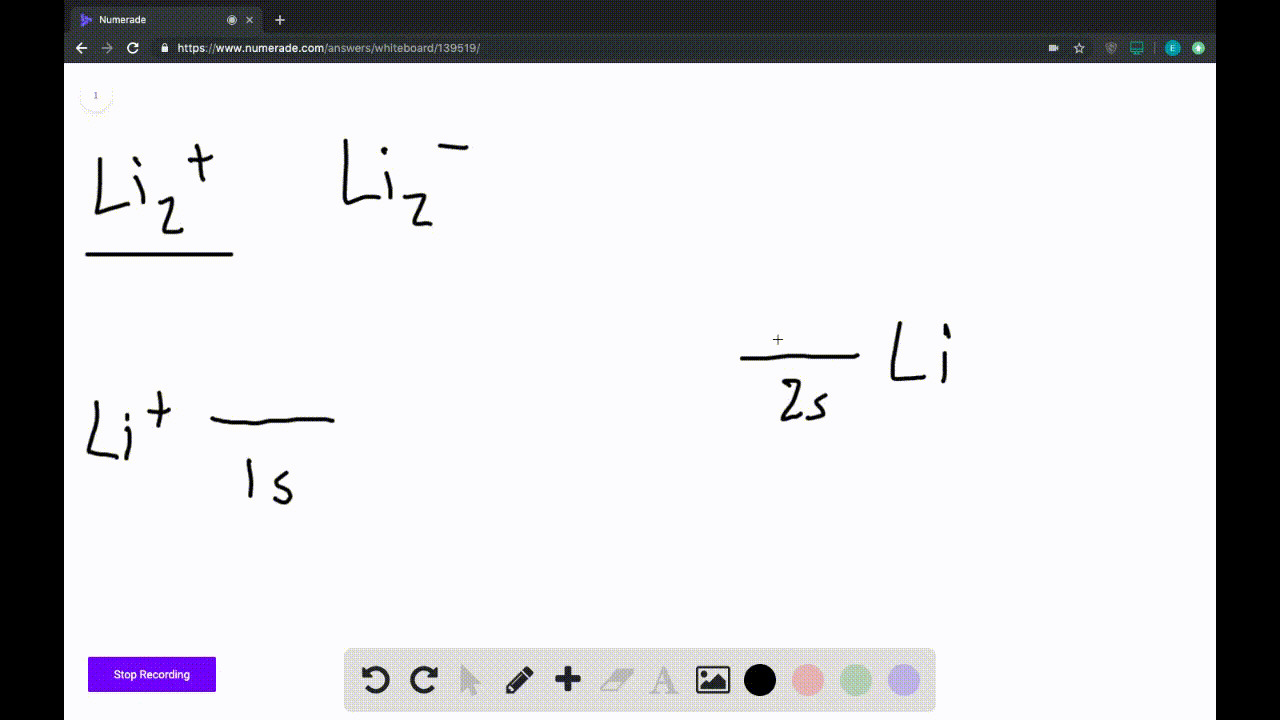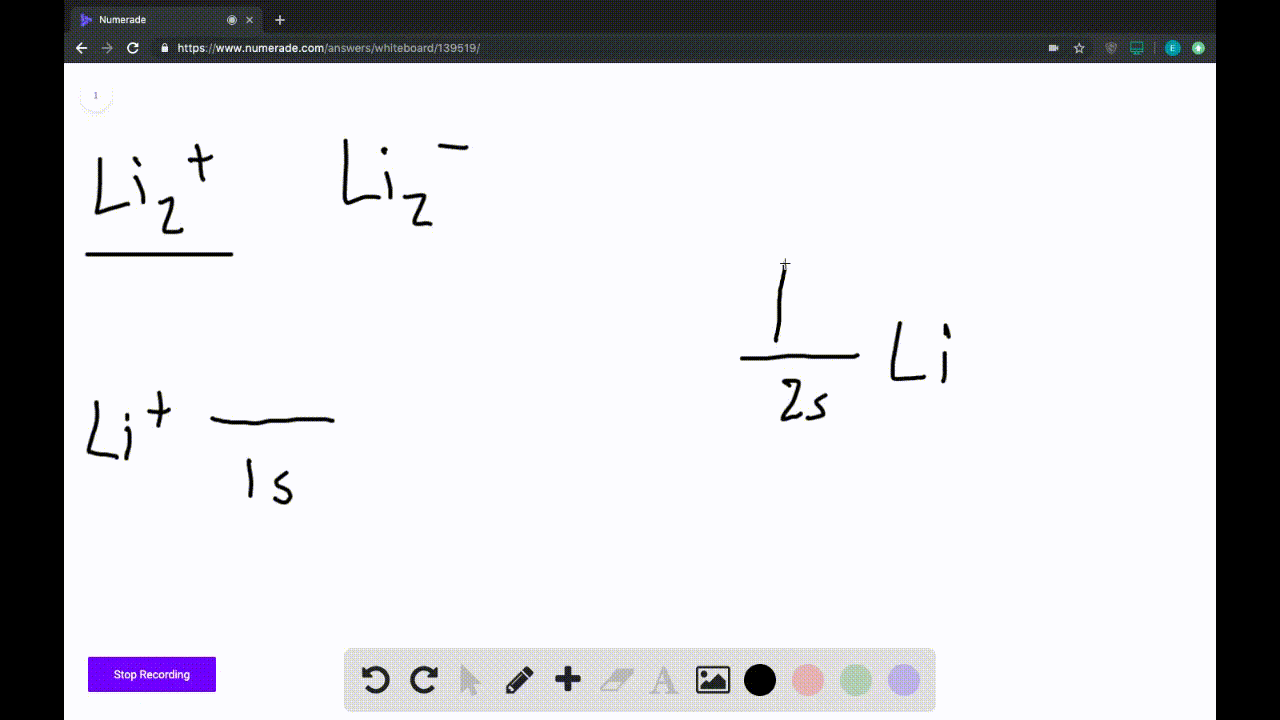
Draw an MO energy diagram and predict the bond order of Li2+and Li2 ... Okay, so this problem test our understanding of the molecular arbiters. They asked us to draw the molecular arbitral diagram for the elysium two plus and we see um two miners. So before we draw the diagram first we need to know the electron configurations of the issue. Alicia electron configuration we know is what has to and to s warm. So actually each lithium atom has to up tools that is ... (Get Answer) - Use the drawing of the MO energy diagram to predict the ... 1. Draw an Molecular Orbital energy diagram and predict the bond order of Li2+ and Li2-. Do you expect these molecules to exist in the gas phase?( Li2+: 2 is subscript, + is superscript)2. Draw an Molecular Orbital energy diagram and predict the bond... SOLVED:Draw an MO energy diagram and predict the bond order of Li2+and ... Chemical Bonding - Intro. In chemistry, a chemical bond is a lasting attraction between atoms that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between opposite charges, dipole-dipole attraction (see also polarization (chemistry)), or the sharing of electrons as in covalent bonding. Use the drawing of the mo energy diagram to predict the bond | Quizlet To construct an MO diagram, we have to know the electron configurations of the constituting atoms. Lithium is a second-period element with the configuration 1 s 2 2 s 1 1s^22s^1 1 s 2 2 s 1. The internal electrons don't really participate in bonding, thus we will only consider the valence electrons.
mastering chemistry help? Use the drawing of the MO energy diagram to ... Likewise Li2^- has an MO scheme of σ(↑↓) σ*(↑) and also a bond order of 0.5 and again should be observed in the gas phase. For reasons I won't go into Li2^- should be less stable than Li2^+. How to Make the Molecular Orbital Diagram for Li2- (Bond Order ... The bond order of Li2- is also calculated and the meaning of this numbe... This video discusses how to draw the molecular orbital (MO) diagram for the Li2- ion. Energy level diagram for Molecular orbitals - Class Notes 2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals.. Bond Order = ½ ( N b - Na). The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero. 3) Relative stability of molecule in terms ... Use the drawing of MO energy diagram for CO to predict the bond order ... Draw an MO energy diagram for HCl. Predict the bond orderand make a sketch of the lowest energy bonding molecularorbital... Draw an MO energy diagram and predict the bond order of Li2 + and Li2 -. Do you expect these molecules to exist in the g... Draw an MO energy diagram and predict the bond order of Be2 + and Be2-. Do you expect these ...

















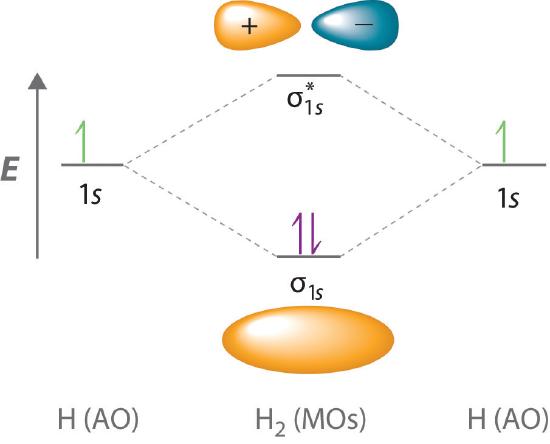


0 Response to "39 use the drawing of the mo energy diagram to predict the bond order of li2+."
Post a Comment