39 bohr diagram for aluminum
BOHR DIAGRAM FOR ALUMINUM - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... topblogtenz.com › sodium-bohr-modelHow to draw Bohr diagram for Sodium(Na) atom - Topblogtenz Steps to draw the Bohr Model of Sodium atom. 1. Find the number of protons, electrons, and neutrons in the Sodium atom. Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei.
Bohr Model For Aluminum - EMMAMICHAELS.COM Kesimpulan dari Bohr Model For Aluminum. A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in the diagram depicts the atom as a positively charged. Draw nucleus of aluminum atom. My daughter needed to create a model of an element for her 9th grade science class so we sat down ...
Bohr diagram for aluminum
bohr diagram for aluminum Diagram dot cross aluminium oxide chloride ... bohr diagram for aluminum Diagram dot cross aluminium oxide chloride potassium If you are looking for Ehlers Aluminum you've visit to the right place. We have 9 Pictures about Ehlers Aluminum like Ehlers Aluminum, Aluminium (Al) and also Aluminium (Al). Read more: Ehlers Aluminum History of the periodic table - Wikipedia The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties.In the basic form, elements are presented in order of increasing atomic number, in the reading sequence. Then, rows and columns are created by starting new rows and inserting blank cells, so that rows and columns show … 3.5: Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 3.5. 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell.
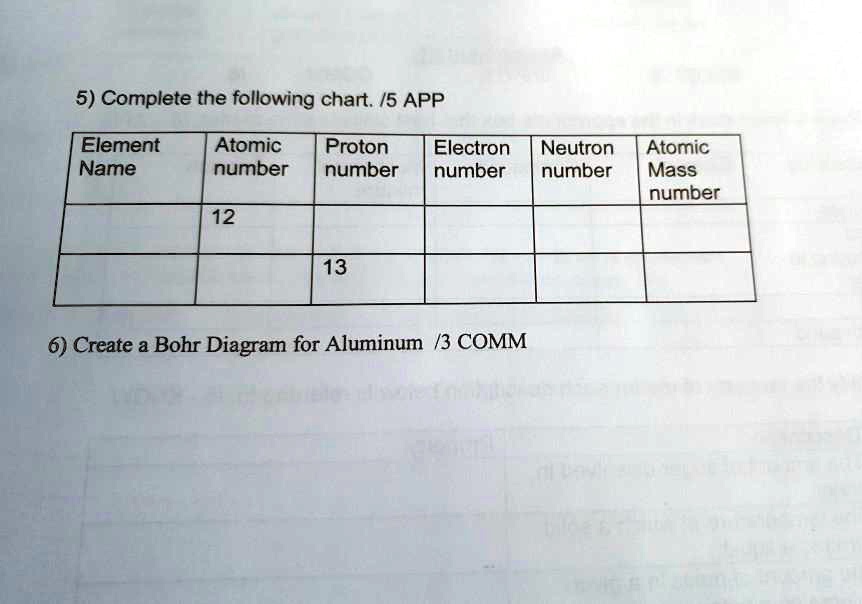
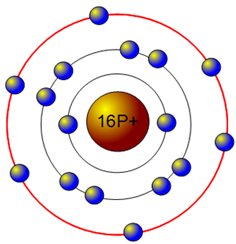
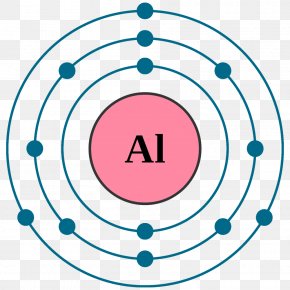
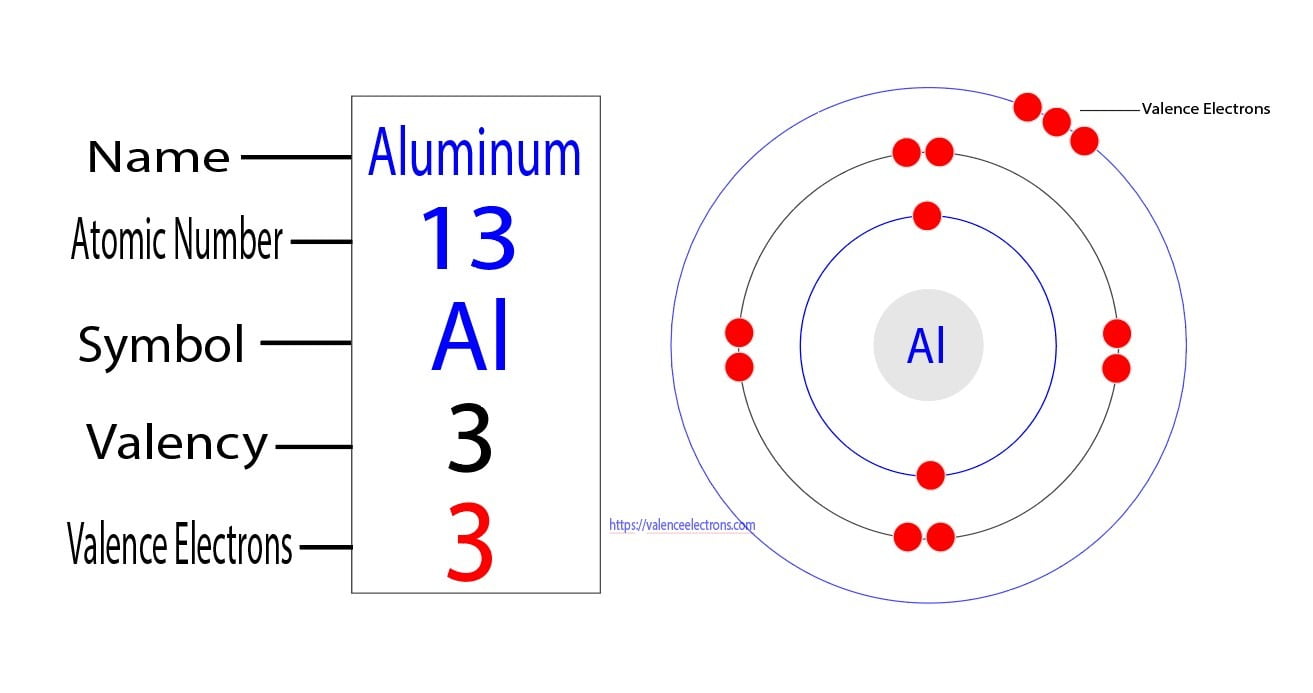
Bohr diagram for aluminum. How to draw Bohr diagram for Helium(He) atom - Topblogtenz From the Bohr diagram of an atom, we can easily find the number of valence electrons in an atom by looking at its outermost shell. So, we have to find a valence electron in the Helium atom, for this, look at its Bohr diagram. Bohr’s diagram of … topblogtenz.com › nitrogen-bohr-modelNitrogen Bohr Model - How to draw Bohr diagram for Nitrogen(N ... The Bohr Model of Nitrogen(N) has a nucleus that contains 7 neutrons and 7 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Nitrogen contains 5 electrons that also called valence electrons. topblogtenz.com › how-to-draw-bohr-model-diagramHow to draw a Bohr model of an atom | Bohr-Rutherford Diagrams Bohr model or Rutherford–Bohr diagram, presented by Niels Bohr and Ernest Rutherford in 1913. Bohr model of an atom consists of a small nucleus that contains protons and neutrons, this nucleus is surrounded by different electron shells or energy levels where electrons are revolved in a definite circular path “similar to the structure of the Solar System“. How to draw Bohr diagram for Aluminum (Al) - Topblogtenz According to the Bohr diagram of Aluminum, the outer shell is M-shell which contains only three valence electrons. Properties of Aluminum It has a great ability to reflect light. It appears as silvery grey metallic. It has a boiling point of 2470 °C and melting of 660.32 °C. It has three electrons beyond a stable noble gas configuration.
Silicon - Wikipedia Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor.It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen ... Light - Wikipedia Light or visible light is electromagnetic radiation within the portion of the electromagnetic spectrum that is perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 terahertz, between the infrared (with longer wavelengths) and the ultraviolet (with shorter … Aluminum(Al) electron configuration and orbital diagram - Valenceelectrons Aluminum orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. The first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. The 1s orbital is now filled with two electrons. Bohr Model of all Elements (Diagrams + Chart Inside) - Periodic Table Guide Bohr Model of all Elements (Diagrams + Chart) Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
topblogtenz.com › helium-bohr-modelHow to draw Bohr diagram for Helium(He) atom - Topblogtenz From the Bohr diagram of an atom, we can easily find the number of valence electrons in an atom by looking at its outermost shell. So, we have to find a valence electron in the Helium atom, for this, look at its Bohr diagram. Bohr’s diagram of Helium has only one electron shell (K-shell ). Aluminum Bohr Diagram A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram. en.wikipedia.org › wiki › LightLight - Wikipedia Light or visible light is electromagnetic radiation within the portion of the electromagnetic spectrum that is perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 terahertz, between the infrared (with longer wavelengths) and the ultraviolet (with shorter wavelengths). How to draw Bohr diagram for Sodium(Na) atom - Topblogtenz The Bohr Model of Sodium(Na) has a nucleus that contains 12 neutrons and 11 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sodium contains only 1 electron that also called valence electron.
How to Draw the Bohr-Rutherford Diagram of Aluminum - YouTube Aluminum has 2 electrons in its first shell, 8 in its second and 3 in its third.Check me out:
en.wikipedia.org › wiki › MuonMuon - Wikipedia The positive muon, in this context, can be considered a pseudo-isotope of hydrogen with one ninth of the mass of the proton. Because the mass of the electron is much smaller than the mass of both the proton and the muon, the reduced mass of muonium, and hence its Bohr radius, is very close to that of hydrogen.
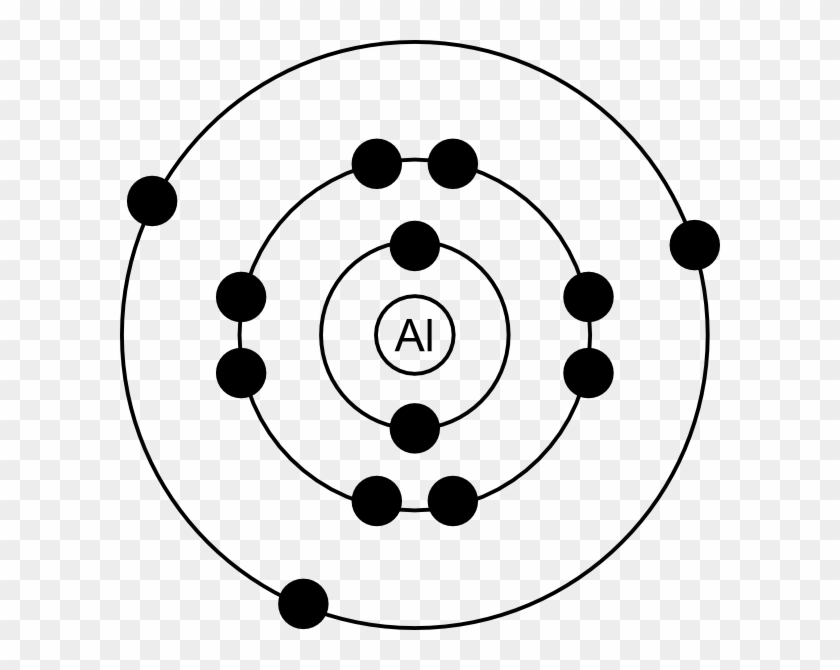
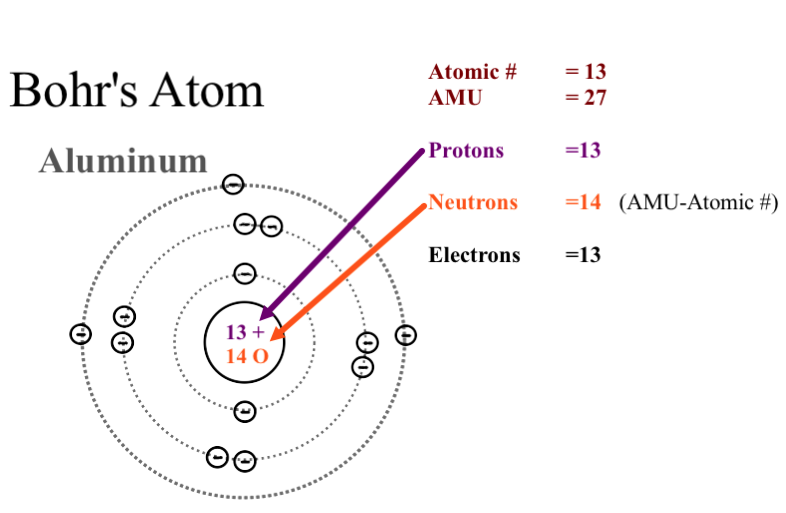
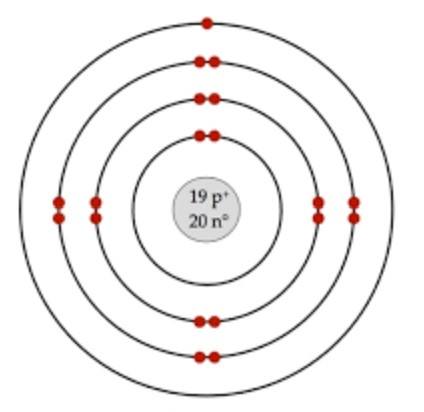
Aluminum Bohr Model - Learnool Aluminum Bohr Model The bohr model of aluminum contains a nucleus having 13 protons and 14 neutrons in the center, and around this nucleus, there are three electron shells containing 13 electrons. Steps Here's how you can draw the bohr model of aluminum step by step. Step #1: write protons, neutrons, and electrons of aluminum atom
Atomic Structure (Bohr Model) for Aluminum (Al) - YouTube In this video we'll look at the atomic structure and Bohr model for the Aluminum atom (Al). We'll use a Bohr diagram to visually represent where the electron...
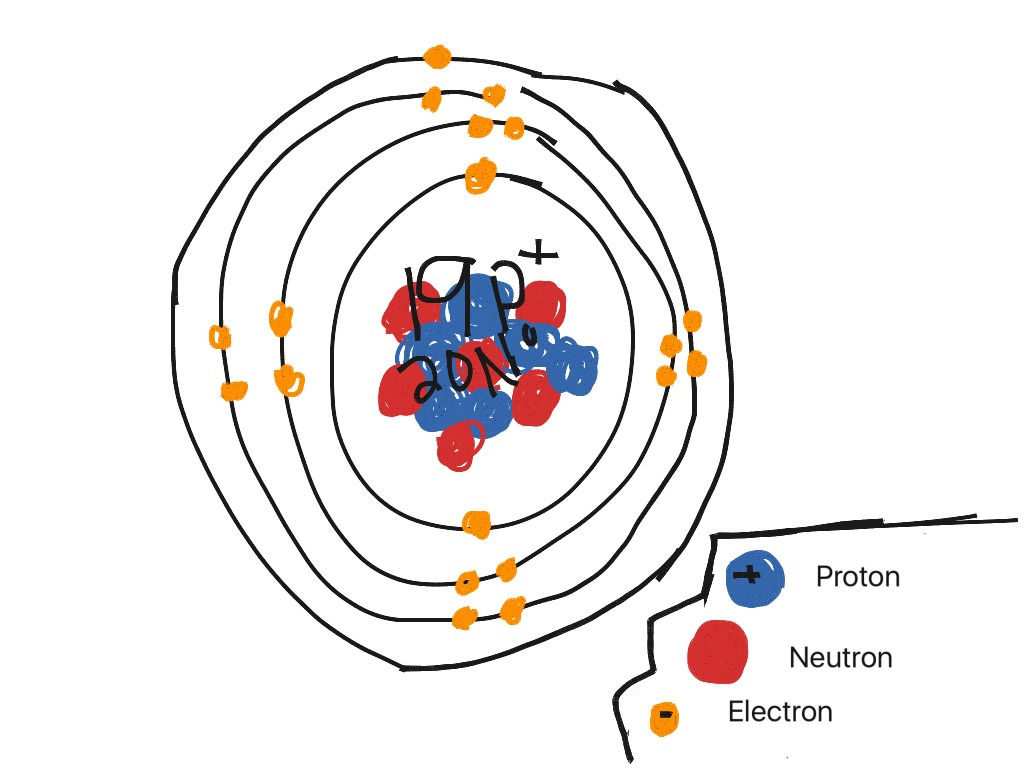
Drawing Bohr Rutherford Diagrams for Ions- Aluminum.docx View Drawing Bohr Rutherford Diagrams for Ions- Aluminum.docx from Science CSE303 at London School of Business and Finance. / 8 marks Bohr Rutherford Diagram :IONS For how to complete the drawing
How to draw Bohr diagram for Nitrogen(N) atom - Topblogtenz Also check: Bohr model for each elements of Periodic table. Find the Valence electron of Nitrogen through its Bohr diagram. Valence electrons are found in the outermost shell of an atom and they can take participate in the formation of a chemical bond. These electrons have more energy compare to the inner shell electrons.
What Is The Bohr Model For Aluminum? Aluminum is neutral and its atomic number is 13, hence, the number of protons and electrons available for its Bohr diagram is also 13. A Visual Depiction. A Bohr model is a way of visually depicting the structure of an atom of a particular element. An atom is the main component of an element and the determinant of an element.
Muon - Wikipedia A muon (/ ˈ m juː ɒ n / MYOO-on; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 e and a spin of 1 ⁄ 2, but with a much greater mass.It is classified as a lepton.As with other leptons, the muon is not thought to be composed of any simpler particles; that is, it is a fundamental particle.
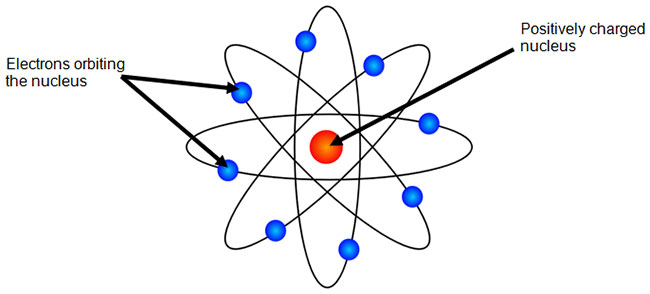
What is Bohr Model? (See Bohr Diagrams of all 118 Elements) Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. This is similar to the planets revolving around the sun, except that the orbits are non-planar. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy. Each orbit (or shell) can hold a certain number ...
Aluminum (Al) Orbital diagram, Electron configuration, and Valence ... The Aluminum orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the remaining one electron in the 3p orbital. An orbital diagram for a ground-state electron configuration of an Aluminum atom is shown below-
Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms.
What is the Bohr diagram for aluminum? - Answers The bohr model of aluminum is 13 protons and 14 neutrons. What is bohr diagram for the element vanadium? Here: Where are the atomic numbers of an atom on the Bohr diagram? The atomic...
How to find Valence electrons | Various method and Examples Q – The electron configuration of aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1, Find it’s valence electrons? Answer – The electron configuration of aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. ... From the Bohr diagram of an atom, we can easily find the number of valence electrons in an atom by looking at its outermost shell.
en.wikipedia.org › wiki › SiliconSilicon - Wikipedia Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor.
How to draw a Bohr model of an atom | Bohr-Rutherford … Bohr model or Rutherford–Bohr diagram, presented by Niels Bohr and Ernest Rutherford in 1913. Bohr model of an atom consists of a small nucleus that contains protons and neutrons, this nucleus is surrounded by different electron shells or energy levels where electrons are revolved in a definite circular path “similar to the structure of the Solar System“.
Aluminum (Al) Orbital diagram, Electron configuration, and … The first shell of Aluminum has 2 electrons and the outer shell or valence shell of Aluminum has 3 electrons, hence, the number of valence electrons in the Aluminum atom is 3. The orbital diagram for Aluminum is drawn by following three principles – the Aufbau principle, Hund’s principle, and Pauli’s exclusion principle.
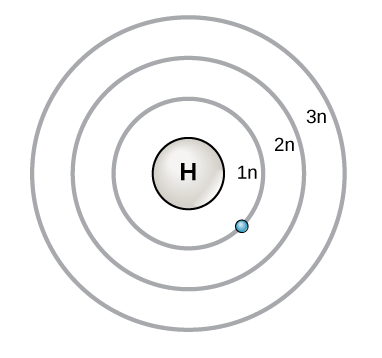
PDF How to Draw Bohr Diagrams Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
3.5: Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 3.5. 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell.
History of the periodic table - Wikipedia The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties.In the basic form, elements are presented in order of increasing atomic number, in the reading sequence. Then, rows and columns are created by starting new rows and inserting blank cells, so that rows and columns show …
bohr diagram for aluminum Diagram dot cross aluminium oxide chloride ... bohr diagram for aluminum Diagram dot cross aluminium oxide chloride potassium If you are looking for Ehlers Aluminum you've visit to the right place. We have 9 Pictures about Ehlers Aluminum like Ehlers Aluminum, Aluminium (Al) and also Aluminium (Al). Read more: Ehlers Aluminum





















0 Response to "39 bohr diagram for aluminum"
Post a Comment