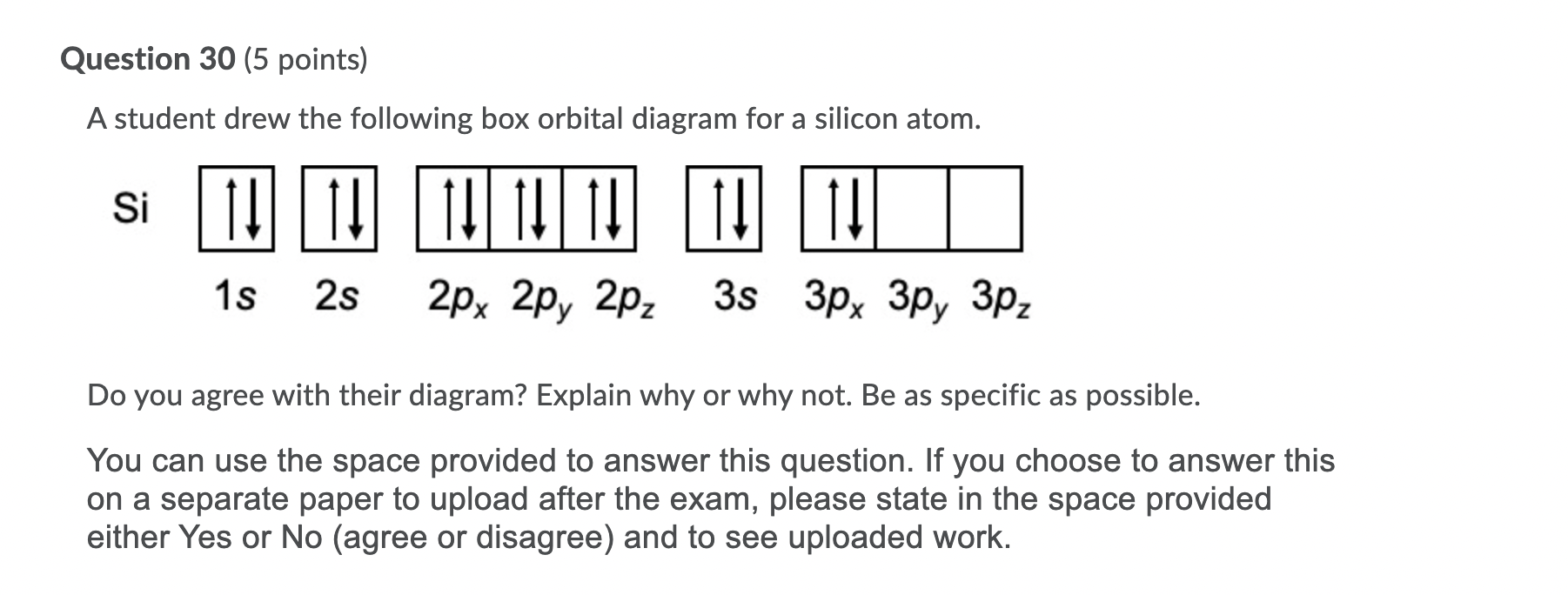
40 orbital diagram for si
DOC Name Draw the orbital diagram for the following elements: Oxygen (O) Titanium (Ti) Silicon (Si) Copper (Cu) For each of the following elements, identify if the electron configuration is correct or incorrect. If it is incorrect, give the fix to the configuration. Carbon (C) = 1s22s22p2. Sulfur (S) = 1s22s22p63p6 Orbital Filling Diagram For Sulfur Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital.
Solved Draw the orbital diagram for the hybridized Si ... Draw the orbital diagram for the hybridized Si atoms in SiH2O and SiO? Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
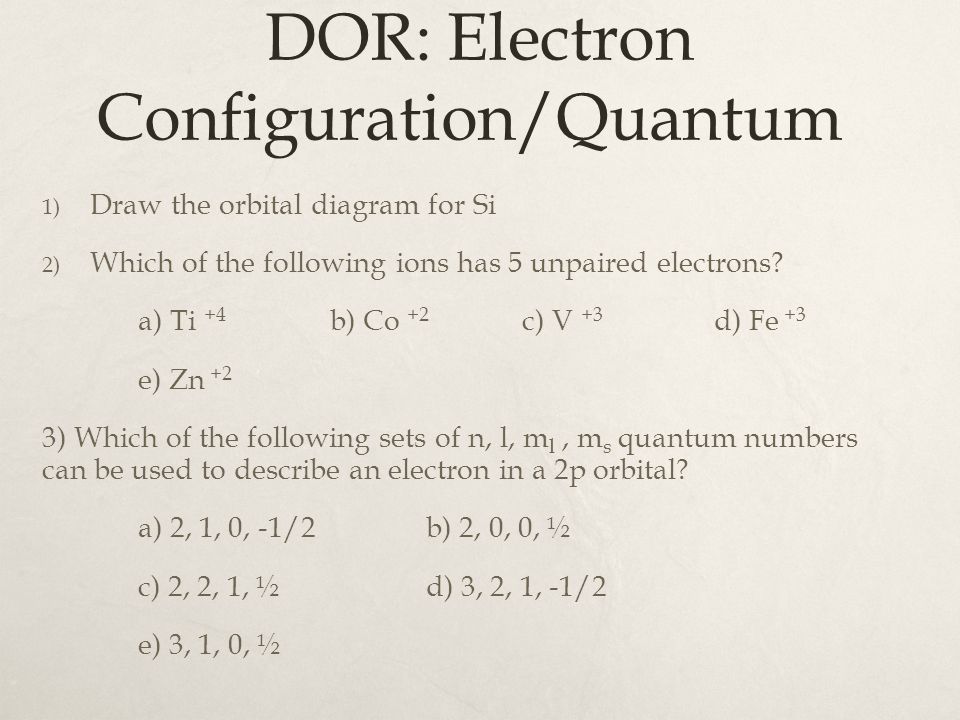
Orbital diagram for si
Nickel(Ni) electron configuration and orbital diagram Atomic Orbital Diagram for Nickel (Ni) Nickel(Ni) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of nickel is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 4s 2. PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? Germanium(Ge) electron configuration and orbital diagram The 3p orbital is now full. So, the next two electrons will enter the 4s orbital and ten electrons will enter the 3d orbital. The 3d orbital is now full. So, the remaining two electrons enter the 4p orbital. Therefore, the germanium(Ge) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 2. How to write the orbital diagram for ...
Orbital diagram for si. How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Silicon Electron Configuration (Si) with Orbital Diagram Jan 26, 2021 · Silicon Orbital Diagram. Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair. Orbital Diagrams Flashcards - Quizlet Si (Silicon) What element is represented by this orbital diagram? F (Fluorine) What element is represented by this orbital diagram? P (Phosphorus) What element is represented by this orbital diagram? V (Vanadium) What element is represented by this orbital diagram? Pauli Exclusion Principle. 2 electrons in the same orbital must have opposite ... Orbital Diagram For Selenium - schematron.org In writing an. Answer to orbital diagram for selenium home / study / science / chemistry / chemistry questions and answers / Orbital Diagram For Selenium. here is the electronic configuration. Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4.Selenium (Se) has an atomic mass of Find out about its chemical and physical properties, states, energy ...
Solved Identify the element corresponding to the orbital ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 92% (13 ratings) Transcribed image text: Identify the element corresponding to the orbital diagram and select all the valence electrons. Ar ΑΙ B Be с Mg Ne o S Si 11111111111111LL 1s 2s 2p 3s 3p. Silicon Orbital Diagram - ViralListClub.com If you look at a Periodic Table silicon is in the orbital 3p2. It has the symbol Si. Silicon and Its Use the orbital diagram to find the third and eighth electrons. Silicon Si is a crystalline blue-grey solid with a metallic appearance. This time we have collected a handy of orbital diagrams with various types in high definition. PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Orbital Diagram For Beryllium The MO diagram is the diagram as a whole. A molecular orbital diagram, Beryllium has an electron configuration 1s 2 2s 2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in diberyllium, whereas there are no p orbitals in the valence level of hydrogen or helium. The orbital filling diagram of ...
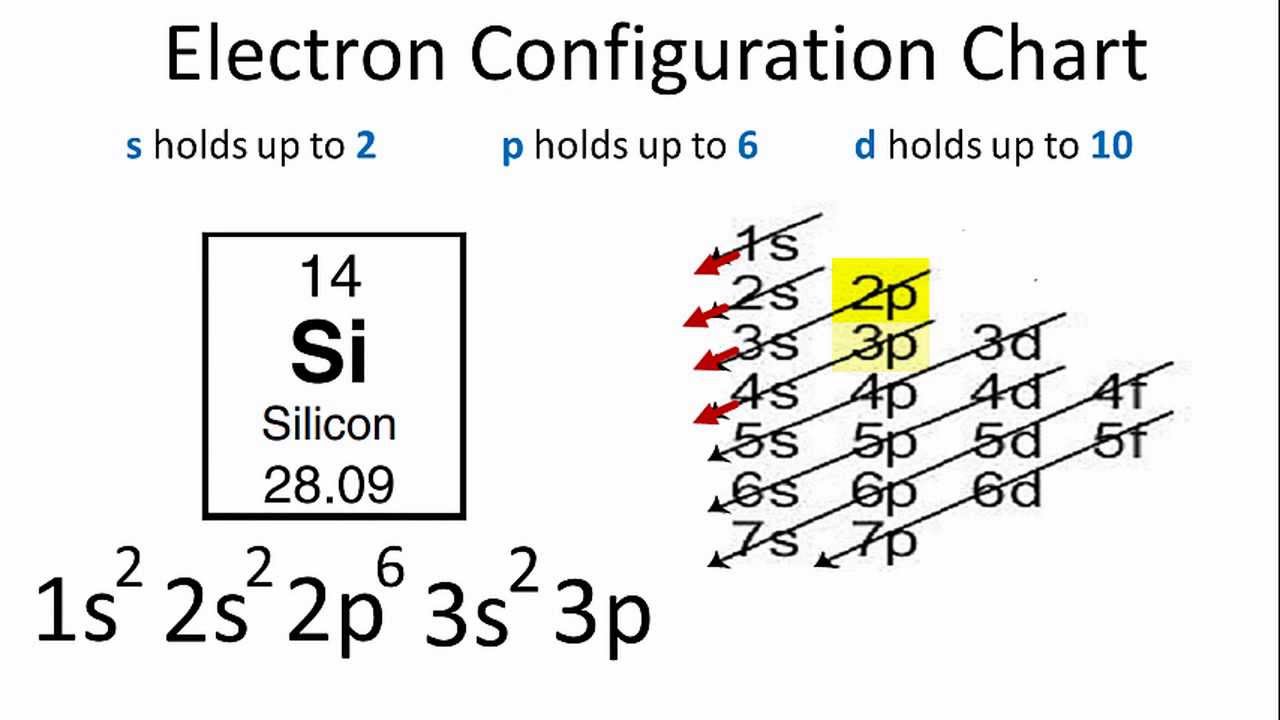
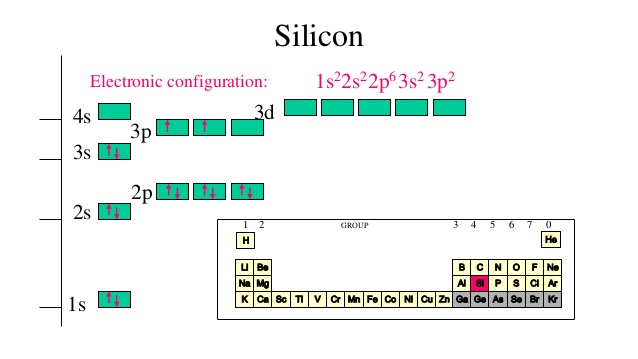
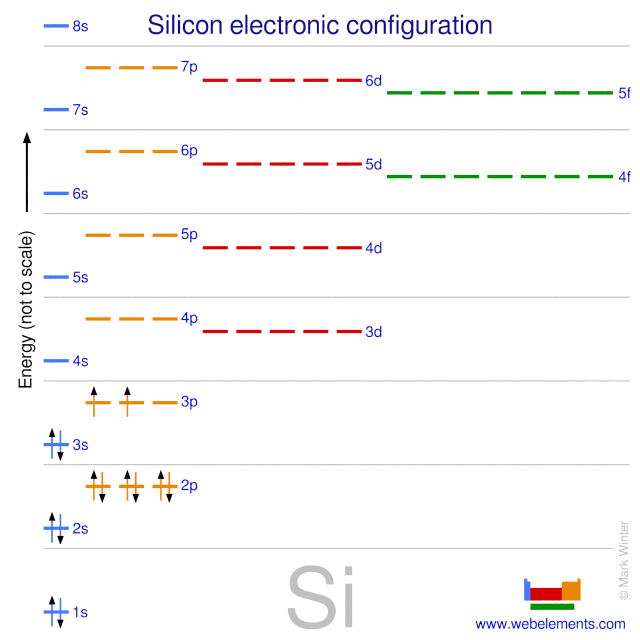
Solved The following orbital diagram corresponds to the ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: The following orbital diagram corresponds to the element _________. A.P B. Si C. S D. Se E. Cl The following orbital diagram corresponds to the element __________. Silicon Electron Configuration | Orbital Diagram For Silicon (Si) Jan 26, 2022 · Silicon Orbital Diagram Well, the orbital diagram of Electron Configuration is the best source of information if you are willing to study the chemical bonding of silicon. This is a kind of qualitative diagram that explains the whole combining process of the element in the best possible manner. Hund's Rule and Orbital Filling Diagrams | Chemistry for ... In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal energy level and sublevel. Electrons are indicated by arrows inside the circles. Silicon(Si) electron configuration and orbital diagram Orbital diagram for silicon(Si) Silicon(Si) excited state electron configuration. Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of silicon is 1s 2 2s 2 2p 6 3s 2 3p 2. The valency of the element is determined by electron configuration in the excited state.
Beryllium Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining .. Beryllium has an electron configuration 1s22s2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in .Feb 23, · A quiz solution for Inorganic Chemistry in which students were prompted to draw the ...
PDF Quantum Numbers Worksheet with Orbital Diagrams CHEMISTRY 151 - ORBITAL DIAGRAMS AND ELECTRON CONFIGURATIONS KEY 1. Write a complete orbital diagram and electronic configuration for germanium, Ge. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 T T Q Q Q T T E 2 & 3. Write abbreviated electronic configurations for the following. Predict whether each atom or ion is paramagnetic or diamagnetic. a. zinc, Zn ...
Silicon (Si) Orbital diagram, Electron configuration, and ... Silicon (Si) Electron Configuration Orbital diagram for Silicon. The orbital diagram simply represents the arrangement of electrons in the different orbital of an atom, it uses an arrow to represent the electrons, every orbital(one box) contains a maximum of 2 electrons. There are three rules followed for constructing the orbital diagram for an ...
Electron Configuration for Silicon (Si) - UMD In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
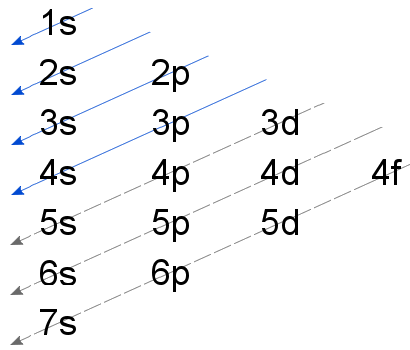
6.4 Electronic Structure of Atoms (Electron Configurations ... The 1 s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p, 3 s, and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms.
Electron configuration of silicon (Si), orbital diagram, and ... Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom.
What is the orbital diagram for silicon? - Studyrankersonline In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital.
Worksheet 4-1 Orbital Diagrams. 1. Fill in the electron configurations for the elements given in the table. Use the orbital filling diagrams to complete the table. ... Si 1s22s22p63s23p2 Ti 1s22s22p63s23p64s23d2 Electron Configurations for Some Selected Elements. Orbital filling. Element. 1s. 2s. 2px. 2py. 2pz. 3s. Electron configuration ...
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
Orbital Diagrams - Concept - Chemistry Video by Brightstorm Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital.
PDF Orbital Diagrams Activity - LCPS 9. There are 3 rules for making orbital diagrams. Use the information you gathered to complete each rule. Circle the word that correctly fills in the blank a. Aufbau Principle- An electron will occupy the highest / lowest energy orbital that can receive it. b. Hund's Rule- Orbitals of equal energy must each contain one / two
Germanium(Ge) electron configuration and orbital diagram The 3p orbital is now full. So, the next two electrons will enter the 4s orbital and ten electrons will enter the 3d orbital. The 3d orbital is now full. So, the remaining two electrons enter the 4p orbital. Therefore, the germanium(Ge) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 2. How to write the orbital diagram for ...
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
Nickel(Ni) electron configuration and orbital diagram Atomic Orbital Diagram for Nickel (Ni) Nickel(Ni) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of nickel is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 4s 2.




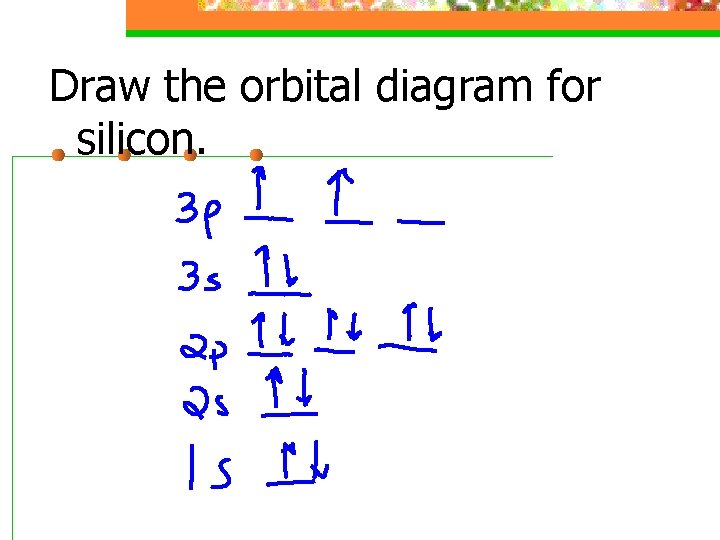




/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)


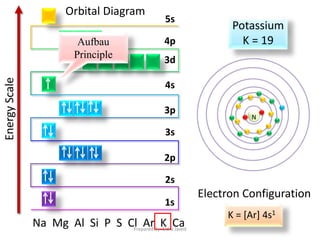




:max_bytes(150000):strip_icc()/aufbauexample-56a129555f9b58b7d0bc9f48.jpg)









0 Response to "40 orbital diagram for si"
Post a Comment