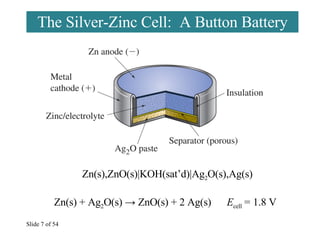
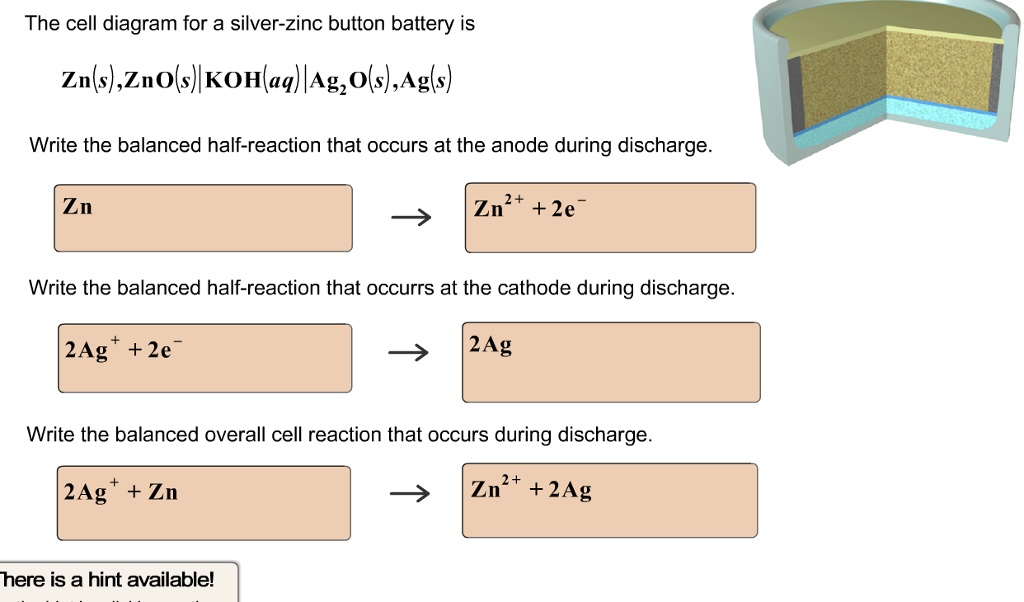
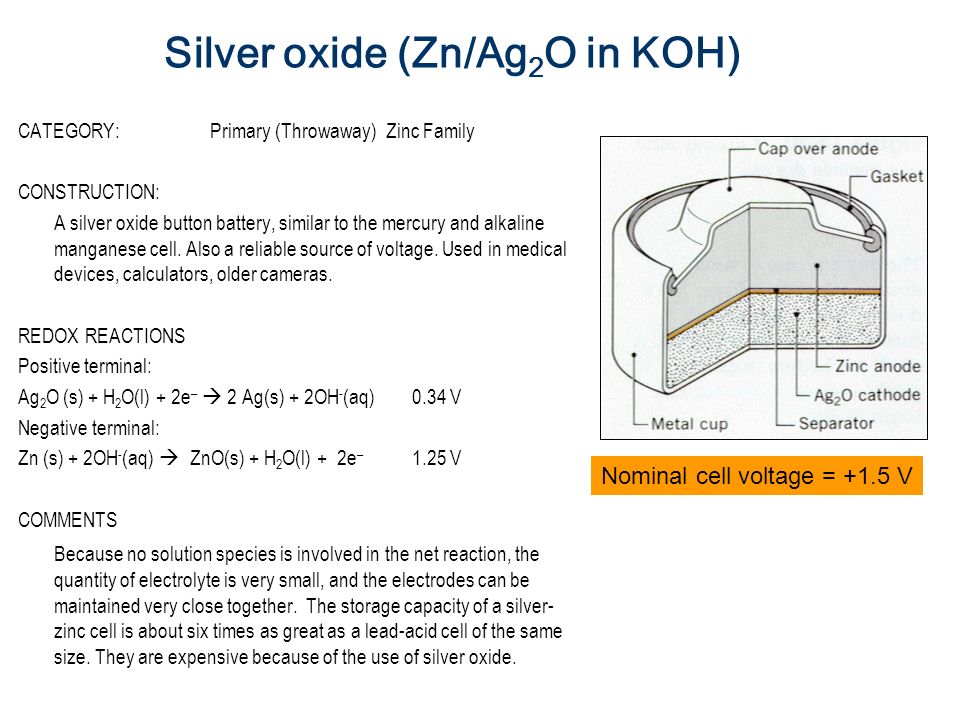
40 the cell diagram for the reaction occurring in silver-zinc button batteries is
Future batteries, coming soon: Charge in seconds, last months a The battery is still lithium-ion like the one found in your smartphone, but it uses silicon instead of graphite in the anodes. It works with current smartphones and uses biological semiconductors made from naturally occurring organic compounds known as peptides - short chains of amino acids - which... (PDF) Leaching of Metals from Waste Silver Oxide-Zinc Button Cell... button cell batteries; silver; Zinc; Aspergillus niger; culture supernatant; two-step bioleaching. 1. Introduction. The varying volumes of spent medium from 5 to 20 mL in a 50 mL flask were employed for the. study. We added 0.10 g waste battery powder and flasks were placed at 60.
The cell diagram for the reaction occurring in silver-zinc button... Write the half-reaction equation that involves zinc. Write the balanced equation for the net cell reaction. And finally, calculate the value of E cell. Not sure how to approach this problem. help is greatly appreciated.
The cell diagram for the reaction occurring in silver-zinc button batteries is
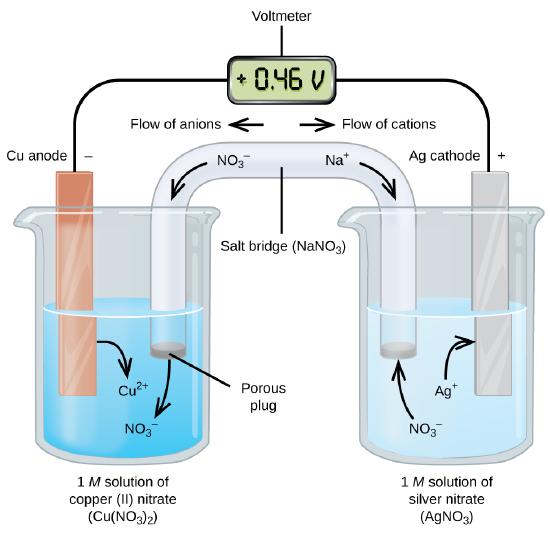
The Simplest Math Problem No One Can Solve - Collatz Conjecture The Collatz Conjecture is the simplest math problem no one can solve — it is easy enough for almost anyone to understand but notoriously difficult to solve. 17.2 Galvanic Cells - Chemistry The cell notation (sometimes called a cell diagram) provides information about the various species involved in the reaction. One of the simplest cells is the Daniell cell. It is possible to construct this battery by placing a copper A zinc sulfate solution is floated on top of the copper sulfate solution... The Cell Diagram For The Reaction Occurring In Silver Zinc Button... Button batteries are used in wristwatches and medical devices where a constant voltage is required another type of primary battery is the s...
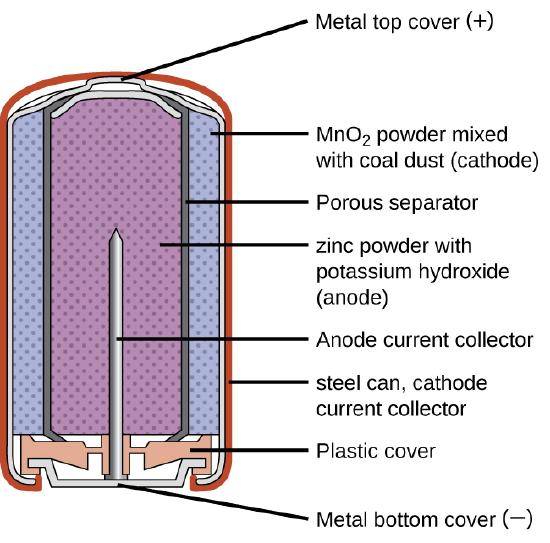
The cell diagram for the reaction occurring in silver-zinc button batteries is. Commercial Galvanic Cells - Chemistry LibreTexts | Button Batteries The half-reactions that occur in an alkaline battery are as follows Although some of the small button batteries used to power watches, calculators, and cameras are miniature alkaline cells In these "button" batteries, the anode is a zinc-mercury amalgam rather than pure zinc, and the... Ch27Cells and Batteries 08 27.2 Primary Cells Batteries such as dry cells, alkaline cells and button cells are non-rechargeable. They go flat when the cell reaction reaches equilibrium. Example: The overall reaction that occurs in the silver-zinc button cell is: Zn(s) + Ag2O(s) + H2O(l) Zn(OH)2(s) + 2Ag(s) Write equations for... Batteries and Cells | Zinc-Carbon Cell The reaction will continue until the supply of zinc is used up. The electrons, with their negative The EMF of the battery is given by E and represents the voltage when the battery is open circuit. Rechargable or Secondary Cells. Rechargeable batteries are rechargeable because the chemical... Introduction to galvanic/voltaic cells (video) | Khan Academy How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current.. And we saw the reaction right over here-- solid zinc plus copper sulfate in solution and water. In this diagram, we picked sodium sulfate as our salt. So for every sulfate molecule, you have sulfate anion.
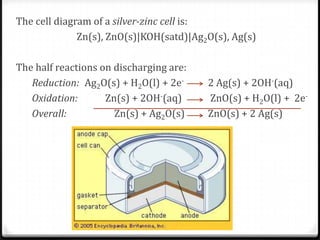
Answered: Zn(s)|ZnO(s)|KOH(aq)||Ag2O(s)||Ag(s)… | bartleby The cell diagram for the reaction occurring in silver-zinc button batteries is. 1. Write the half‑reaction equation that involves silver. Silver zinc battery - Wikipedia A silver zinc battery is a secondary cell that utilizes Silver(I,III) oxide and Zinc. Silver Zinc cells share most of the characteristics of the silver-oxide battery, and in addition, is able to deliver one of the highest specific energies of all presently known electrochemical power sources. SOLVED:The cell diagram for a silverâ€'zinc button battery is Zn... So we can have the following reaction occur from. And mainly here we have to check that we have the same number of species on both sides with the same charge and we see that this is Show the signs of the electrodes and label the anode and cathode. Write the equation for the net cell reaction. The Cell Diagram For The Reaction Occurring In Silver Zinc Button... Strictly speaking a battery consists of two or more electrochemical cells that are connected together. Write the balanced equation for the...
The cell diagram for the reaction occurring in silver-zinc button... Question Purchase it 1) Calculate the cell potential for the following reaction as written at 25.00 °C, given that [Cr2 ] = 0.897 M and [Sn2 ] = 0.0190 M. Standard reduction potentials can be found here. (https (a) Write the half-reaction equation that involves silver. Solved The cell diagram for the reaction occurring in | Chegg.com Write the half-reaction equation which involves zinc. These cookies are necessary for the website to function and cannot be switched off in our systems. They are usually only set in response to actions made by you which amount to a request for services, such as setting your privacy preferences... Electrochemistry: Galvanic Cells and the Nernst Equation Step 2: The Electrochemical Cell. In the movie on the previous page, when a zinc strip is placed in a The following is an electrochemical cell diagram for the reaction shown in the movie above: Zn(s) From the video and the reactions above can you determine which side is the oxidation half-cell and... Corrosion - galvaniccell The reactions occurring are those shown in the diagram. · The zinc electrode is dissolved (corroded or oxidized) and the copper electrode accepts copper atoms from the electrolyte (electroplating or reduction). This process continues as long as the circuit is closed.
Batteries | Free Full-Text | Leaching of Metals from Waste Silver... Keywords: button cell batteries; silver; Zinc; Aspergillus niger; culture supernatant; two-step bioleaching button cell batteries; silver; Zinc The aqua regia method was used for the digestion of waste button cell battery powder. The digested solution was filtered using a 0.45 µm membrane filter...
Energy Density Comparison of Silver-Zinc Button Cells with... Silver-zinc batteries have the highest theoretical specific energy (Wh/kg) and energy The reaction at the AgO cathode involves a two-step oxidation (1.8 V and 1.5 V) of two water molecules to For the other two silver-zinc button cell sizes, due to the higher intrinsic material and capacity density of the...
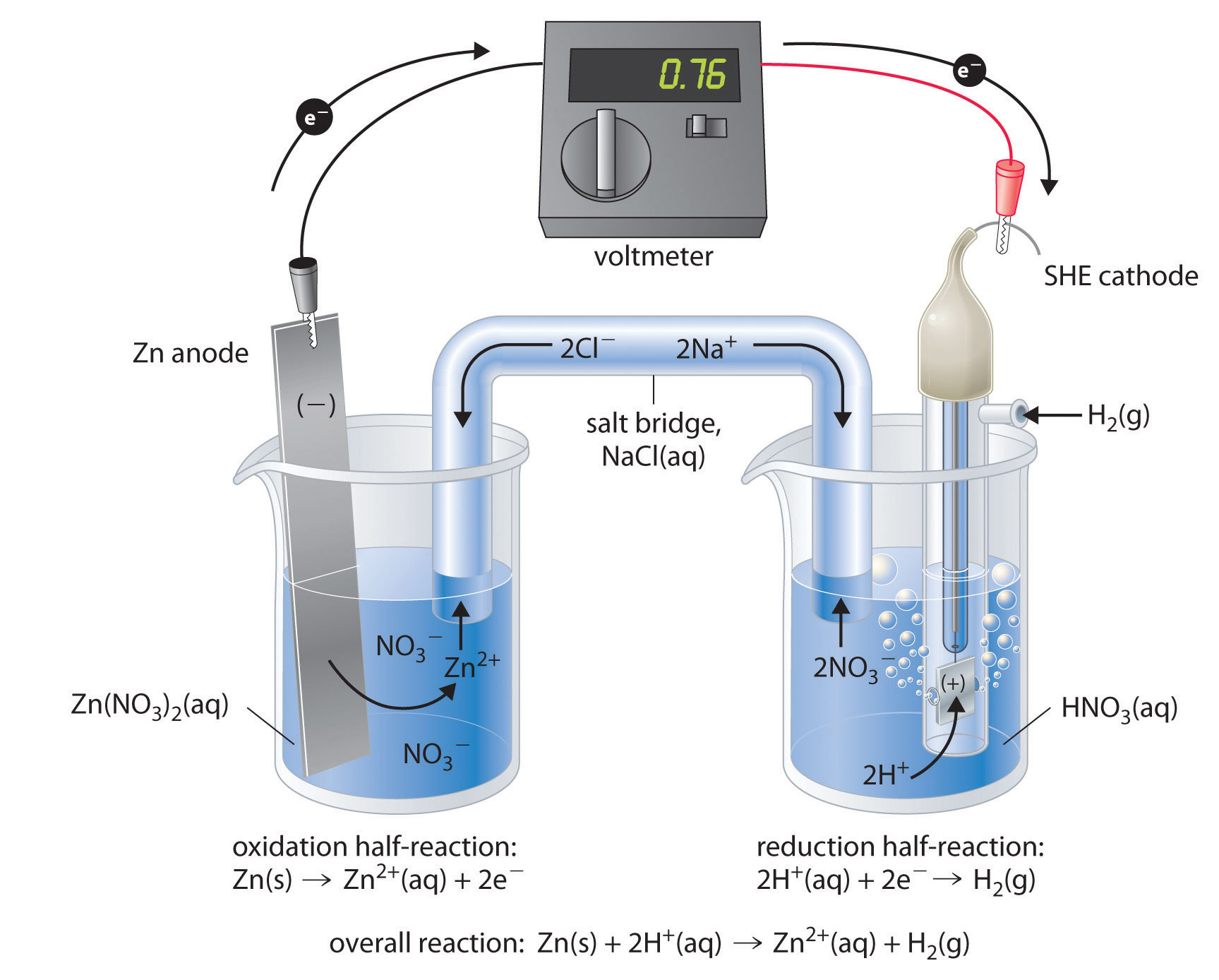
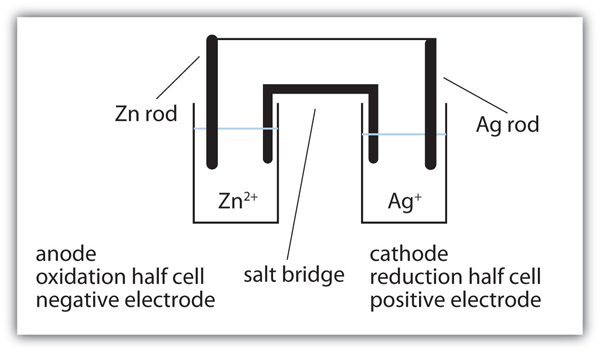
Describing Electrochemical Cells For the reaction of zinc with bromine, the overall chemical reaction is as follows Each half-reaction is written to show what is actually occurring in the system; Zn is the reductant in this reaction (it loses electrons), and Br2 is the oxidant (it gains electrons).
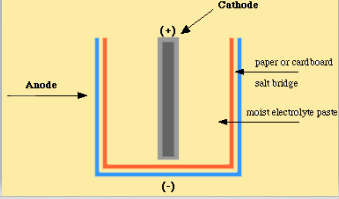
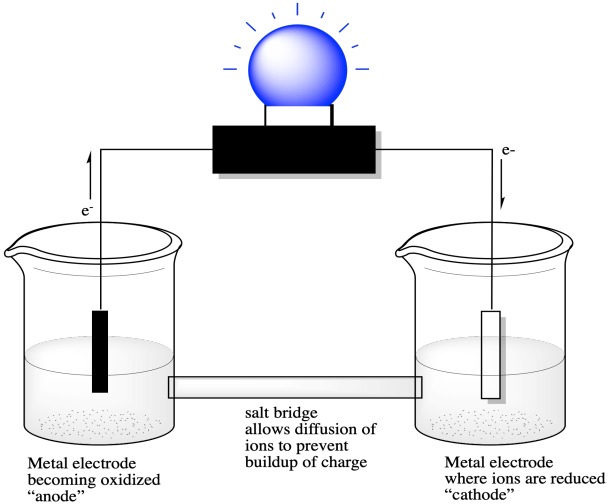
13.3 Galvanic and electrolytic cells | Electrochemical reactions Electrochemical reactions (ESCR4). In Grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper(II) sulfate. In the galvanic cell experiment, make sure that the sodium chloride paste is highly concentrated and fills the U-tube for the best results.
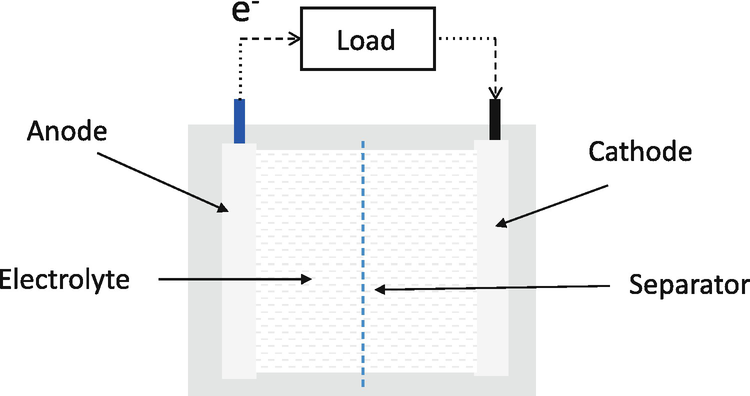
Electrochemical Cells Electrochemical cells which generate an electric current are called voltaic cells or galvanic cells, and common batteries consist of one or more such cells. The letters in parentheses are just reminders that the zinc goes from a solid (s) into a water solution (aq) and vice versa for the copper.
Silver Zinc Batteries - an overview | ScienceDirect Topics For commercial applications, silver-zinc cells and batteries have been and are still used in Silver-zinc batteries are still utilized in a number of critical space programs. Provided below is a partial list When the cell is fully charged and still is under overcharging, the main cell reactions stop due to the...
Batteries - McQuarrie General Chemistry Such dendrites occur in the rapid discharge of a lead storage battery and can lead to an internal short circuit. The cell diagram for the Edison cell, used extensively in car and truck batteries in Europe, is Fe The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)...
26 The Cell Diagram For The Reaction Occurring In Silver Zinc... ...occurring in silver zinc button batteries is znsznoskohaqag2osags a write the half reaction in silver zinc button batteries is question 1 calculate the cell potential for the following reaction as Applications Of Redox Reactions Voltaic Cells Introductory. Solved The Cell Diagram For The...
Galvanic Cell: Definition, Diagram and Working | Science ABC A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. At the end of the reaction, the strip has become heavier and the solution is replete with zinc. The majority of batteries are actually based on lead and lead oxide as anode and cathode...
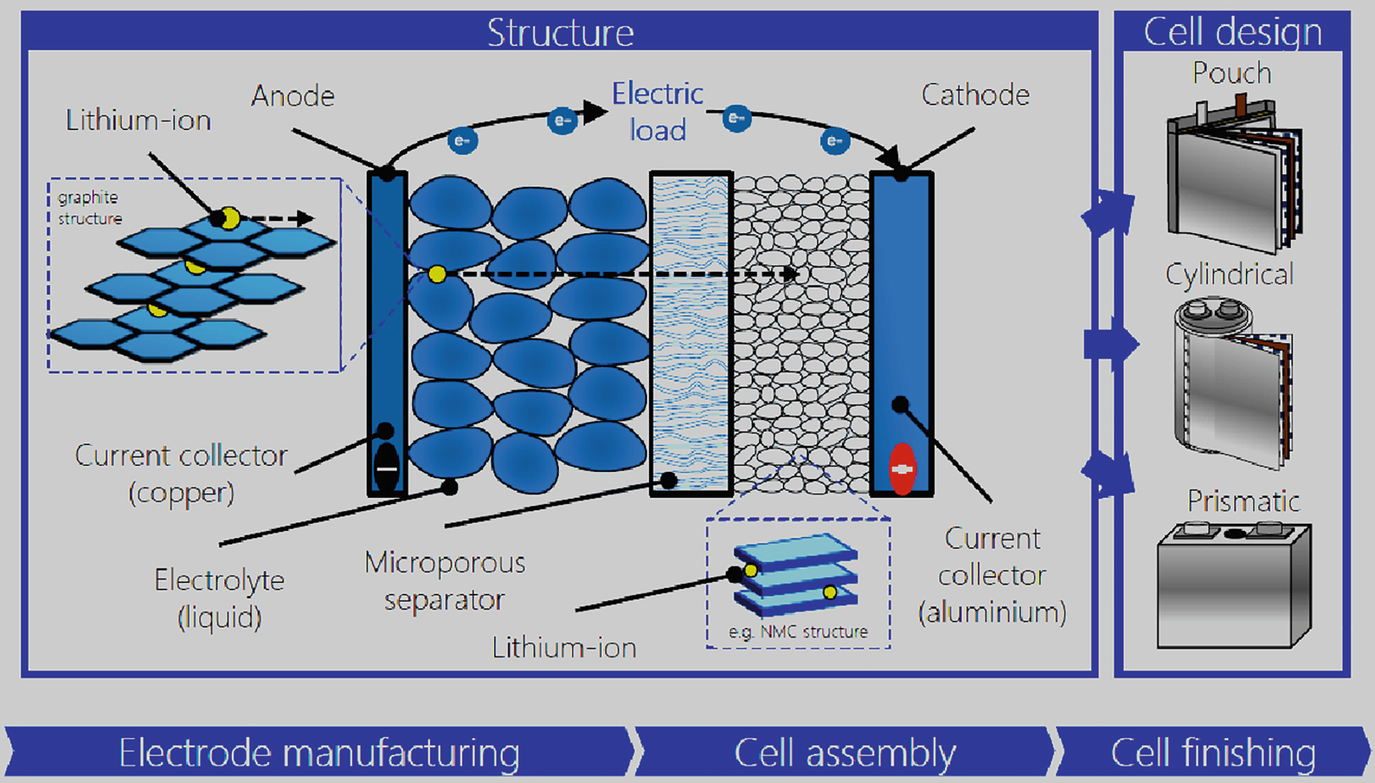
Batteries | Boundless Chemistry | Defining a Dry Cell Common examples of dry-cell batteries include zinc-carbon batteries and alkaline batteries. Key Terms. cathode: The electrode of an electrochemical cell at Chemical reactions occur in every part of the battery to allow for energy storage; the reactions can be described using balanced chemical...
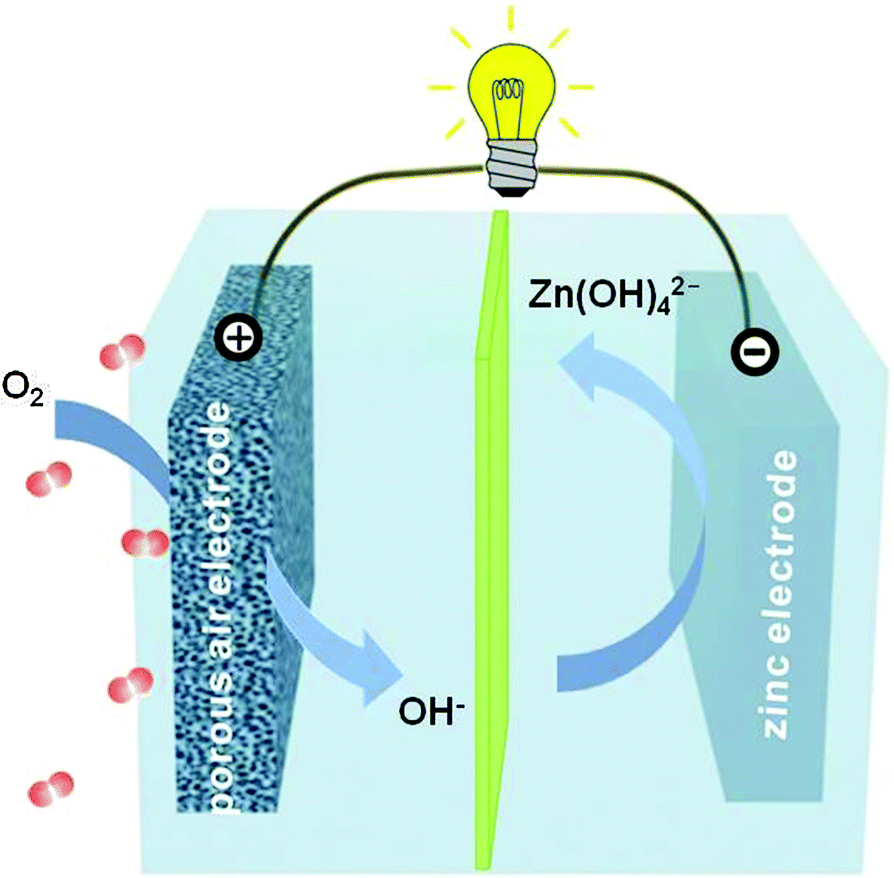
Silver Zinc Cell Silver-zinc batteries have good shockproof and antivibrator properties, low and stable internal resistance, good low temperature ... Trying to recharge a Silver Oxide button cell battery SR916SW using an Arduino. Disclaimer: I am not sure if Zinc / Monovalent ...
Batteries Chemistry Tutorial Examples: Leclanché, Alkaline cell, Lithium battery, Button cell. Maximum voltage that can be delivered by the cell during discharge is equal to the electrode potential for the spontaneous redox reaction.
The Cell Diagram For The Reaction Occurring In Silver Zinc Button... Button batteries are used in wristwatches and medical devices where a constant voltage is required another type of primary battery is the s...
17.2 Galvanic Cells - Chemistry The cell notation (sometimes called a cell diagram) provides information about the various species involved in the reaction. One of the simplest cells is the Daniell cell. It is possible to construct this battery by placing a copper A zinc sulfate solution is floated on top of the copper sulfate solution...
The Simplest Math Problem No One Can Solve - Collatz Conjecture The Collatz Conjecture is the simplest math problem no one can solve — it is easy enough for almost anyone to understand but notoriously difficult to solve.















0 Response to "40 the cell diagram for the reaction occurring in silver-zinc button batteries is"
Post a Comment