36 solidus line phase diagram
What is tie line in ternary phase diagrams ... A phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance can exist. The solid lines identify the temperatures and pressures at which an equilibrium exist between phases. The point at which the lines intersect represents the triple point. How do you read a ternary phase diagram? PDF Chapter Outline: Phase Diagrams Isomorphous system -complete solid solubility of the two components (both in the liquid and solid phases). Binary Isomorphous Systems (I) Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidusline separates solid from liquid + solid α+ L α
Solidus Line - an overview | ScienceDirect Topics Solidus Line In particular, the solidus line defines the temperature below which the phases in the diagram are solid, while the liquidus line defines the temperature above which the phases are completely liquid. From: Advances in Nuclear Fuel Chemistry, 2020 Download as PDF About this page
Solidus line phase diagram
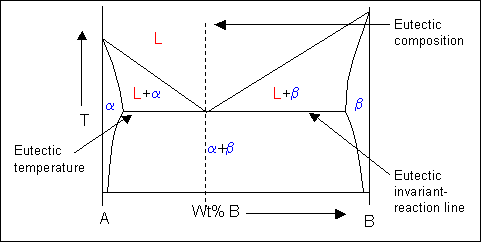
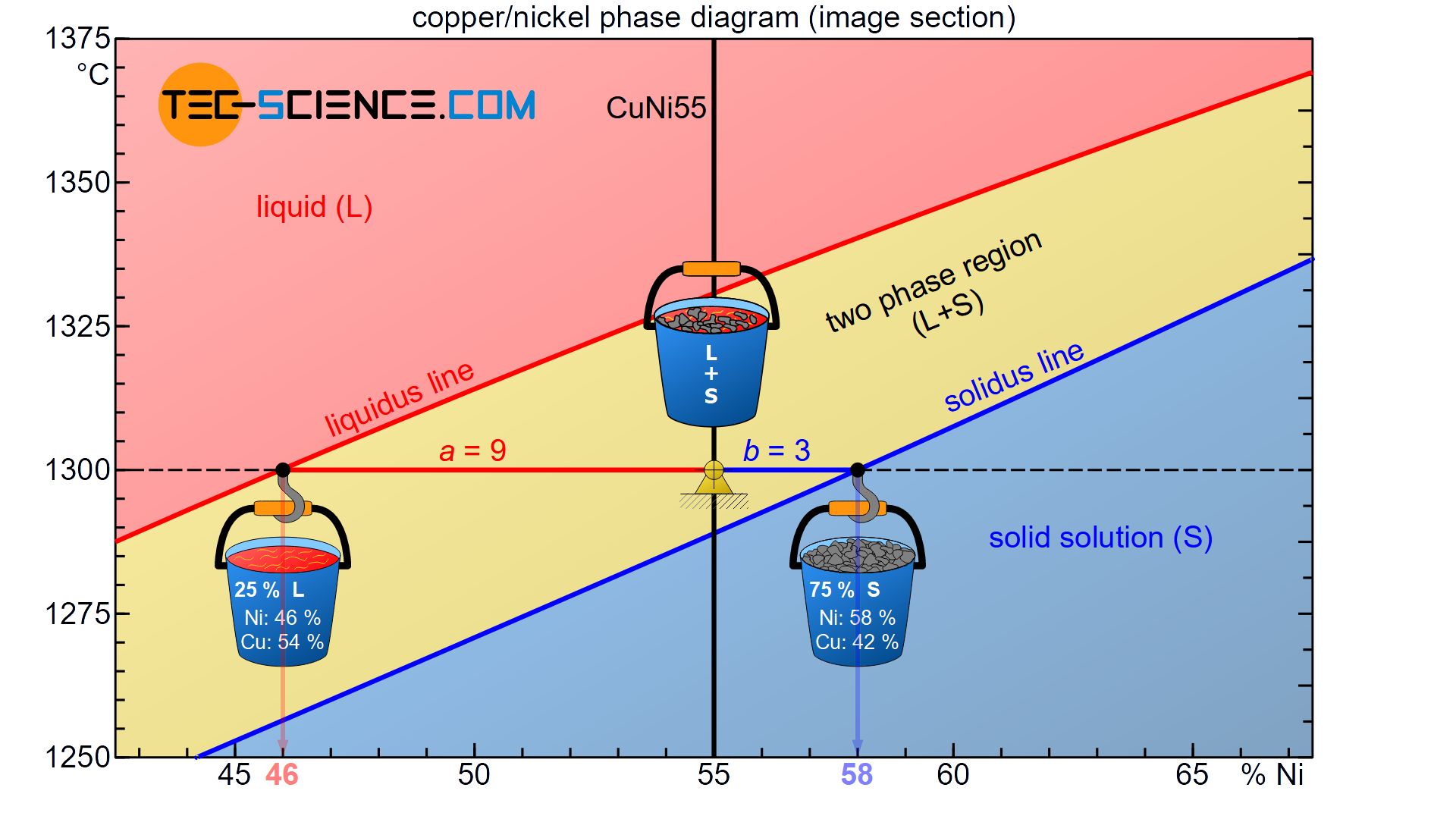
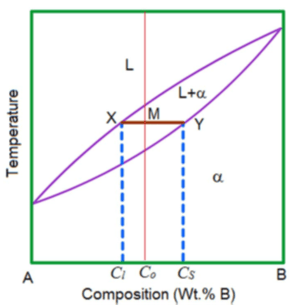
Lecture_14_Phase_Diagrams_MSE_280_S22.pptx - Chapter 10 ... R = C o - C L S = C α - C o 3 . Mass fractions ( wt % ) of each phase : C C S R S W L o 68 . 0 5 . 31 5 . 42 35 5 . 42 Liquid : L C C C C C C S R R W L L o 32 . 0 5 . 31 5 . 42 5 . 31 35 Solid : i.e. 68 % of the mass is liquid and 32 % of the mass is solid EngArc - L - Liquidus, Solidus, Solvus, and Eutectic The solvusis represented by a line on a phase diagram that separates a solid phase from a solid1 + solid2 phase, where solid1 and solid2 are different microstructures. The eutecticis represented by the horizontal line in a eutectic binary phase diagram, connecting the intersections of the solidus and solvus lines from both sides. Phase Diagrams Flashcards | Quizlet Above the following line, liquid phase exist for all compositions in a phase diagram. (a) Tie-line. (b) Solvus. (c) Solidus. (d) Liquidus. c. Following is wrong about a phase diagram. (a) It gives information on transformation rates. (b) Relative amount of different phases can be found under given equilibrium conditions.
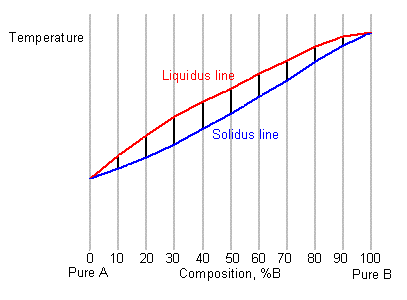
Solidus line phase diagram. PDF The Solidus Line of the Cu-Bi Phase Diagram Journal of Phase Equilibria Vol. 18 No. 2 1997 The Solidus Line of the Cu-Bi Phase Diagram L.-S. Chang, B.B. Straumal*, E. Rabkin, W. Gust, and F. Sommer Max-Planck-Institut für Metallforschung Institut für Metallkunde der Universität Seestrasse 75, D-70174 Stuttgart, Germany (Submitted 18 September 1996; in revised form 8 November 1996) What does the line in the phase diagram represent ... What does the line in the phase diagram represent? General Features of a Phase Diagram The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. 1 the line that connects points A and D separates the solid and liquid phases and shows how the melting point of a solid varies with pressure. Phase diagram - Wikipedia A typical phase diagram. The solid green line applies to most substances; the dotted green line gives the anomalous behavior of water. The green lines mark the freezing point and the blue line the boiling point, showing how they vary with pressure. Phase Diagram: Meaning and Types | Material Engineering This phase diagram consists of two points, two lines and three areas. The two points of the two pure metals A & B. The upper line, obtained by connecting the points showing the beginning of solidification is called liquidius line, and the lower line, determined by connecting the points showing the end of solidification is called the solidus line.
Phase Diagrams (Part 2) - Practical Maintenance As solidus line is a continuous line connecting the melting points of pure metals, the complete solidus line is MFGN. The phase diagram consists of four areas. The area above the liquidus line is a single-phase homogeneous liquid solution, since the two metals are soluble in the liquid state (labeled as Liquid solution). The Solidus Line of the Cu-Bi Phase Diagram | SpringerLink The solid solubility of Bi in Cu single crystals has been experimentally determined. It is shown that the solidus line is a retrograde curve without a monotectic transition. The solid and liquid phases are successfully described with simple thermodynamic models. The experimentally measured maximum solubility of 0.0207 at. % Bi at 975 °C correlates well with that from the model (0.0193 at ... (PDF) The Solidus Line of the Cu-Bi Phase Diagram Liquidus line of the Cu-Bi phase diagram and the metastable miscibility gap in the liquid state. Cu-Bi solidus and liquidus Data Figures - uploaded by Boris Straumal Construction of Solidus Lines of Binary Metal Systems ... The paper presents the calculation results on the construction of solidus lines of phase diagrams for some binary metal systems based on cadmium, zinc and tellurium. The investigations have been carried out using the phase equilibrium thermodynamics and known liquidus lines. By the calculation method the solidus lines of phase diagrams of the Cd-Na, Cd-Tl, Te-Ga, Te-As, Te-Cu and Zn-Sn systems ...
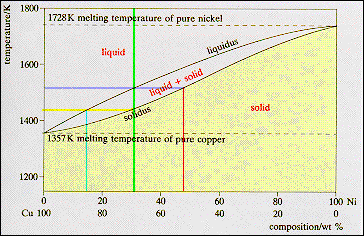
Liquidus Line - an overview | ScienceDirect Topics The phase diagram, therefore, consists of a liquidus line showing a minimum at the eutectic temperature, which is itself marked by a horizontal line. Since the solid phases formed consist simply of pure cadmium or pure bismuth, the solidus lines are coincident with the two vertical temperature axes. Sign in to download full-size image 1.14. Solid Solution Phase Diagram - James Madison University The solid solution phase diagram explains the behavior of chemical solid solution series, such as the transition from high temperature, calcium-rich plagioclase to low temperature sodium-rich plagioclase, or the transition from high temperature magnesium-rich to low temperature iron-rich crystals in ferromagnesium minerals (e.g. olivine, pyroxene). 2 Component Phase Diagrams - Tulane University Solidus- The line separating the field of all solid from that of liquid plus crystals. Eutectic point- the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature must remain constant until one of the phases disappears. A eutectic is an PDF Phase Diagrams, Solid Solutions, Phase Transformations phase diagram A(1100, 60): 1 phase: B(1250, 35): 2 phases: L + Determination of phase(s) present Melting points: Cu = 1085°C, Ni = 1453 °C Solidus - Temperature where alloy is completely solid. Above this line, liquefaction begins. Liquidus - Temperature where alloy is completely liquid. Below this line, solidification begins.
Study on the Solidus Line in Sn-Rich Region of Sn-In Phase ... Binary phase diagrams are important tools for designing desired alloys. In the Sn-rich region of the Sn-In alloy phase diagram, the solidus line appears as a dotted line in current literature plots...
Study on the Solidus Line in Sn-Rich Region of Sn-In Phase ... The solidus line (dotted line in Fig. 1) in the Sn-rich side of the Sn-In phase diagram remained indefinite until now. It was determined by measuring the chemical composition of solid-phase grains in samples annealed at different temperatures in the (liquid + γ) two-phase region.
PDF Lecture30 Phase Diagrams - MIT OpenCourseWare Figure 30-5: The so-called "lens" phase diagram. The upper line is the limit of fsolid! 1 and is called called the solidus curve. The lower line is called the liquidus curve. Figure 30-6: A variation on the lens phase diagram. Consider how the Gibbs phase rule relates to the above phase diagrams. The Gibbs phase rule is: D = C +2¡f
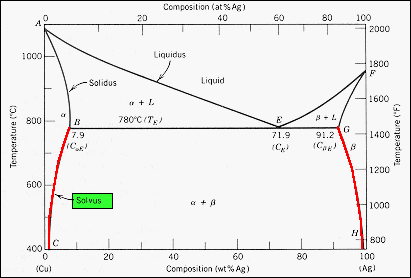
Liquidus vs. Solidus - Lucas-Milhaupt Figure 1 is a phase diagram for the silver-copper binary system. Note that, at the 72% silver, 28% copper composition, liquidus and solidus temperatures are the same. The alloys to the left or right of this eutectic composition do not change directly from solid to liquid, but pass through a "mushy" range where the alloy is a combination of ...
PDF Chapter 9 Phase Diagrams - KSU Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidus line separates solid from liquid + solid Binary Isomorphous Systems (II)
Liquidus vs Solidus - What's the difference? | WikiDiff As nouns the difference between liquidus and solidus is that liquidus is (chemistry|physics) a line, in a phase diagram, above which a given substance is a stable liquid and below which solid and liquid are in equilibrium while solidus is...
What is tie line in phase diagram? - FindAnyAnswer.com The lever rule is a rule used to determine the mole fraction (x i) or the mass fraction (w i) of each phase of a binary equilibrium phase diagram. It can be used to determine the fraction of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus line.
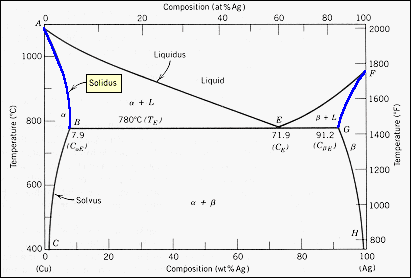
nglos324 - solidus - Princeton The solidus lines on a phase diagram represent the locus of all points that represent the completion of the solidification of a material of given composition as it is cooled in equilibrium from the liquid phase. For the copper-silver binary phase diagram shown two such lines exist, one for the. ( a + L) two phase zone and the other for the ( b ...
PDF Chapter 9: Phase Diagrams - Florida International University in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
Solved Iron-Carbon Phase Diagram 1600 Molten Steel ... Transcribed image text: Iron-Carbon Phase Diagram 1600 Molten Steel Liquidus Line 1400 Solidus Line Liquid and Solid Austenite Solution 1200 Solid Solution Austenite 1000 Upper Transformation Temperature Temperature (°C) 800 Ferrite and Austite Upper Transformation Temperature Austenite and Cementite Lower Transformation Temperature 600 Erre Fermite 400 Ferrite and Pearlite Cementite and ...
materials - Princeton University possible compositions of the alloy. The equilibrium "phase diagram" opposite shows the dependence of the alloys melting point on its composition, and the existence of the single solid phase below the solidus line indicates the complete solid state miscibility of the two elements. The phase diagram
Phase Diagrams Flashcards | Quizlet Above the following line, liquid phase exist for all compositions in a phase diagram. (a) Tie-line. (b) Solvus. (c) Solidus. (d) Liquidus. c. Following is wrong about a phase diagram. (a) It gives information on transformation rates. (b) Relative amount of different phases can be found under given equilibrium conditions.
EngArc - L - Liquidus, Solidus, Solvus, and Eutectic The solvusis represented by a line on a phase diagram that separates a solid phase from a solid1 + solid2 phase, where solid1 and solid2 are different microstructures. The eutecticis represented by the horizontal line in a eutectic binary phase diagram, connecting the intersections of the solidus and solvus lines from both sides.
Lecture_14_Phase_Diagrams_MSE_280_S22.pptx - Chapter 10 ... R = C o - C L S = C α - C o 3 . Mass fractions ( wt % ) of each phase : C C S R S W L o 68 . 0 5 . 31 5 . 42 35 5 . 42 Liquid : L C C C C C C S R R W L L o 32 . 0 5 . 31 5 . 42 5 . 31 35 Solid : i.e. 68 % of the mass is liquid and 32 % of the mass is solid















![PDF] Study on the Solidus Line in Sn-Rich Region of Sn-In ...](https://d3i71xaburhd42.cloudfront.net/a4ed92b1496b4efd5bc6798130321d0b1d87749e/4-Figure3-1.png)

0 Response to "36 solidus line phase diagram"
Post a Comment