36 nacl dissolved in water diagram
Solid-Liquid Phase Equilibria of the Ternary System (NaCl + CH3OH...) According to the experimental data, the phase diagrams and diagrams of physicochemical properties versus sodium is the mass fraction of NaCl in saturated sodium chloride pure water solution and. The calculated thermodynamic functions of NaCl dissolved in the CH3OH-H2O binary system are... SOLVED:Draw a diagram of table salt (NaCl) dissolved in water. we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud.
Is Saltwater acidic, basic, or neutral? (NaCl dissolved in water.) To tell if Saltwater forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules along with the neutralization reaction that...
Nacl dissolved in water diagram
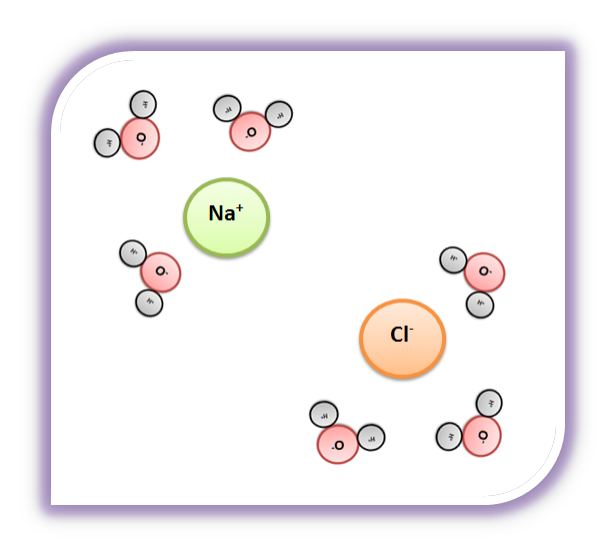
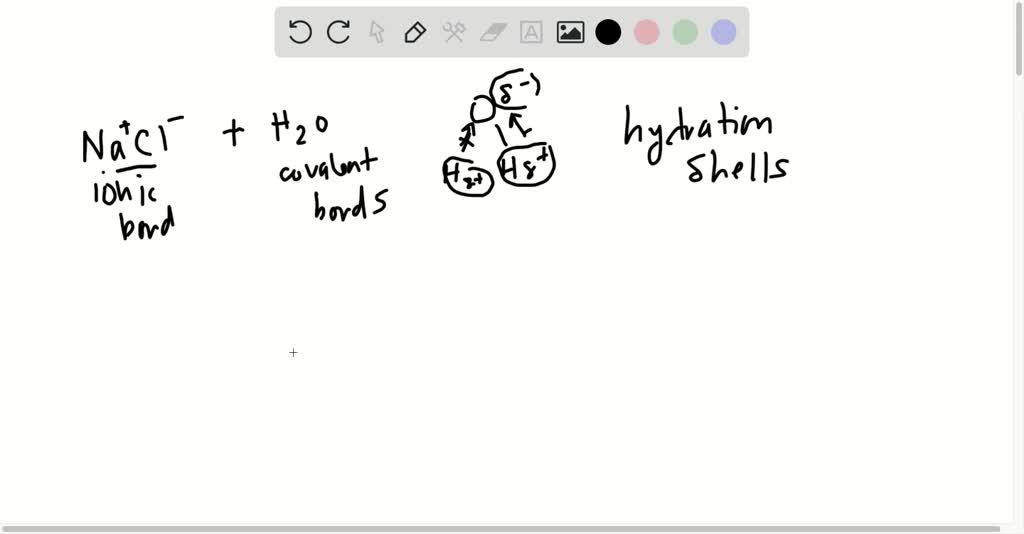
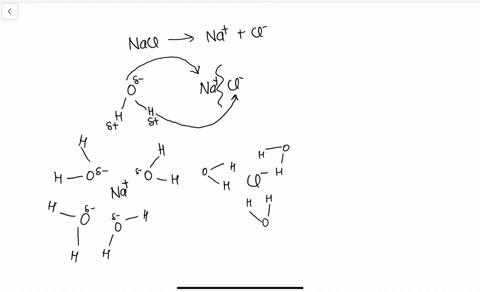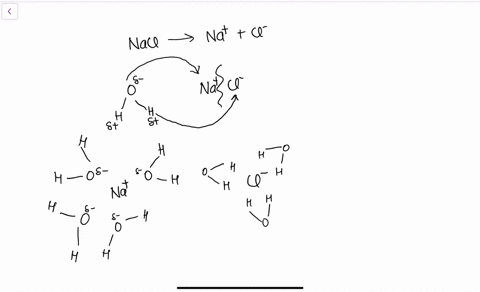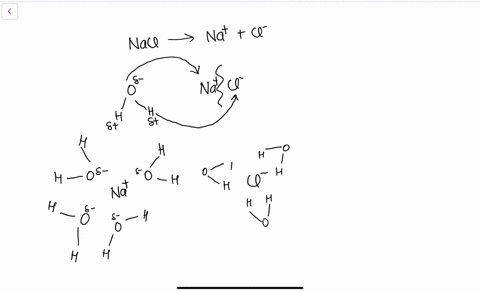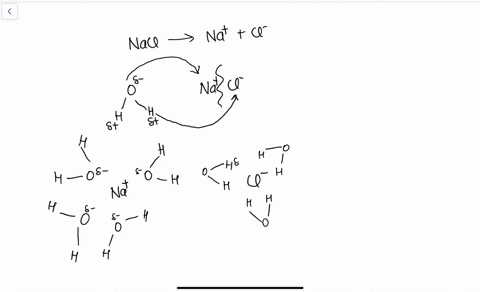
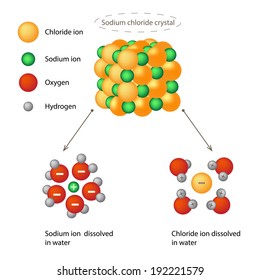
Water's Solvent Properties | Introduction to Chemistry Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Dissociation of NaCl in waterWhen table salt (NaCl) is mixed in water, spheres of hydration form around the ions. Dissolving Salt in Water: Chemical or Physical Change? Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does... What Happens When Salt Is Added to Water? | Sciencing "Salt dissolved in water" is a rough description of Earth's oceans. In chemistry, it results in a solution, as the ionic bond of NaCl is Reviewed by: Lana Bandoim, B.S. The sight of ordinary salt dissolved in water is, in all likelihood, entirely familiar to you, as the phenomenon literally dominates the globe.
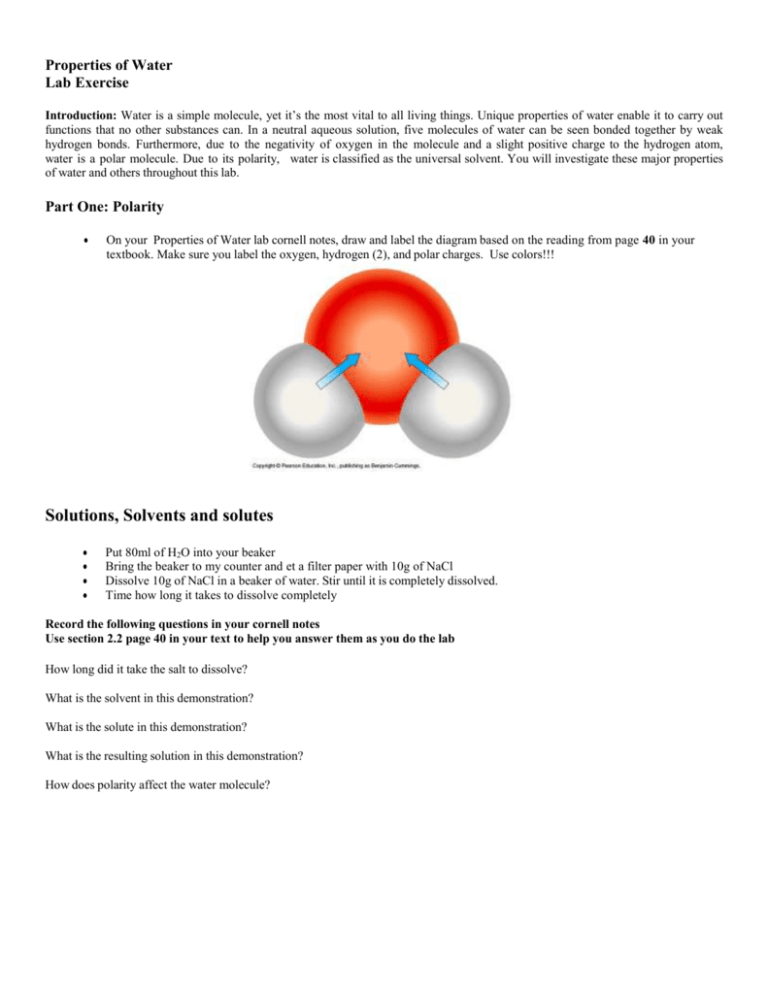
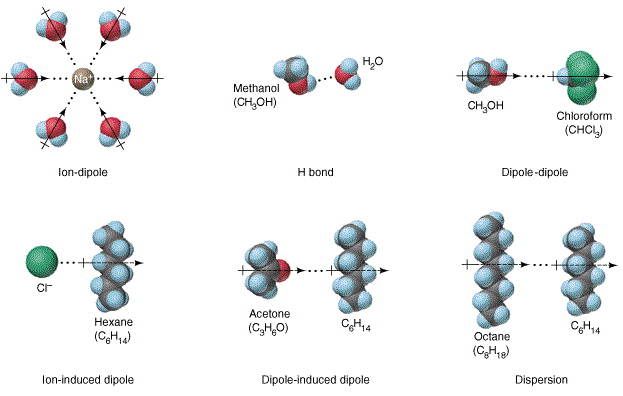
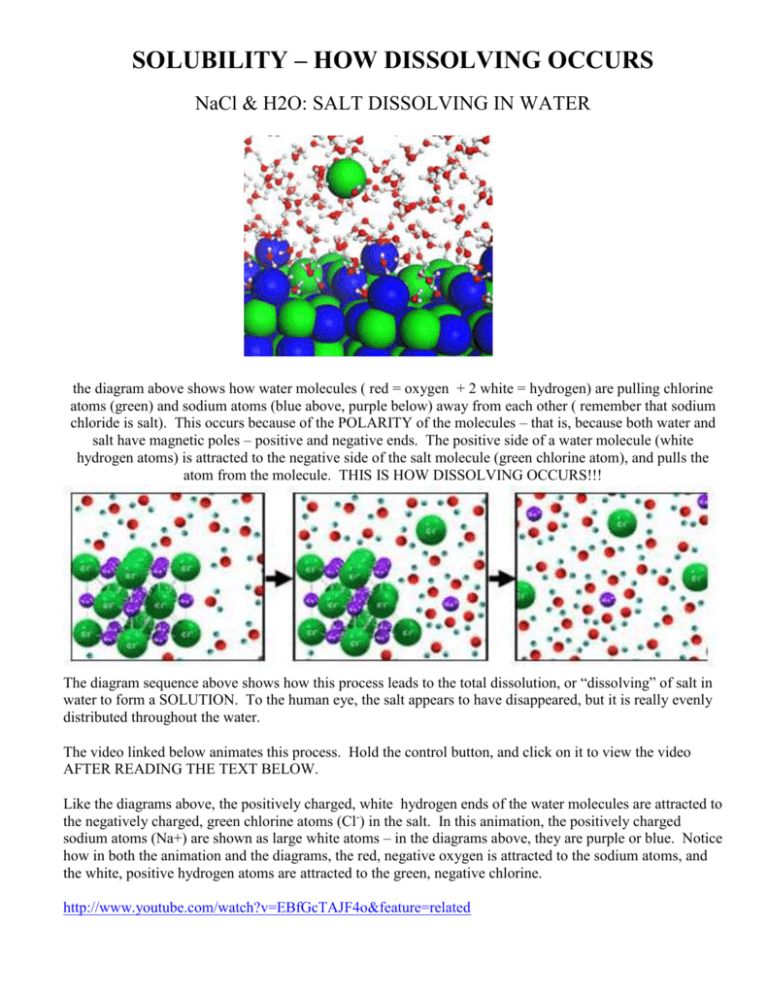
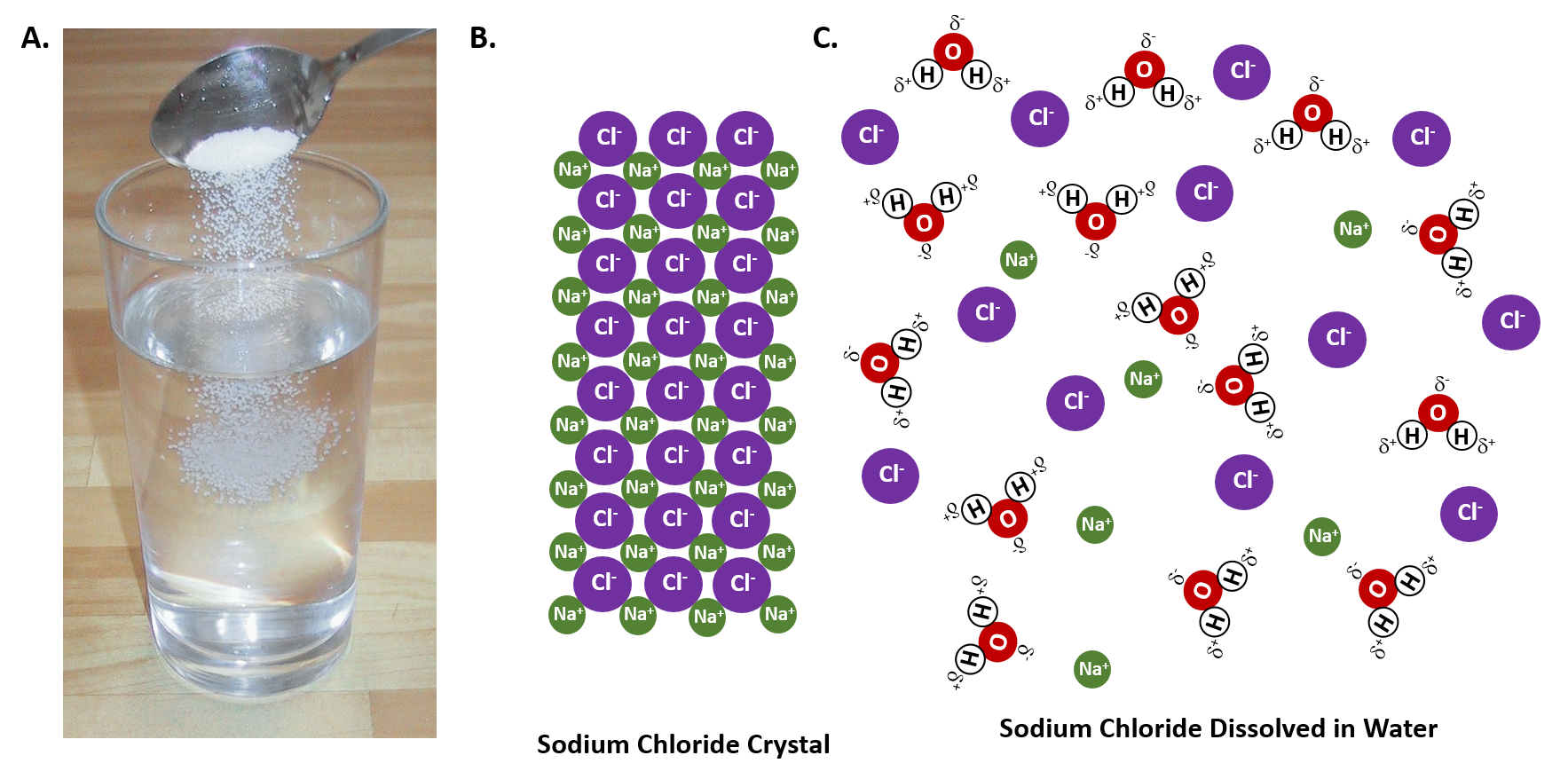
Nacl dissolved in water diagram. Table salt, or NaCl (Sodium Chloride) will dissolve in water. Will NaCl dissolve in water? Yes. sodium chloride is very soluble in water. What is the best method of separation for sand and salt? Dissolve 0,35064 g NaCl in demineralized water, at 20 0C in a volumetric flask. How many grams of NaCl would you dissolve in water to make 50mM NaCl with... NaCl Dissolving in H2O... Entropy at Work? - Science Forums Now onto the salt dissolving in water. The crystal is highly organized and stable..which is in direct contrast to the 2nd law. When submerged in water, the crystal structure breaks apart, changing from an organized state, to a more disorganized state..i.e. increase in entropy. Yes entropy can be quantified... Ions in Water, and Conductivity - HORIBA | NaCl density (W / V) % Salt contains NaCl and KCl, which form electrolytes when dissolved in water, most of which become ions. The relationship between density and conductivity is nearly linear. As is seen in the diagram, however, unlike the low-density zone, the high-density zone does not show an increase in conductivity... Water molecules and their interaction with salt | U.S. Geological Survey This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion (Na or Cl, for example) can At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and...
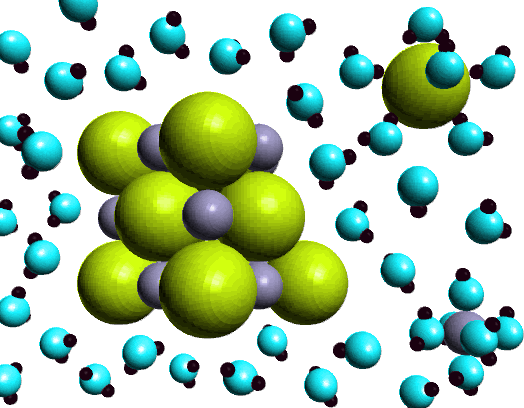
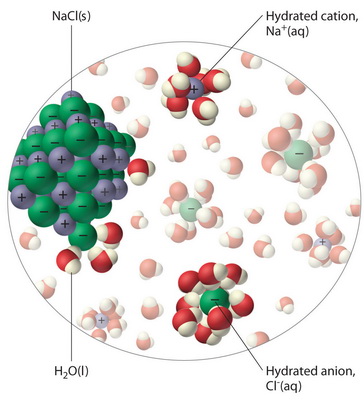
PDF Microsoft PowerPoint - Zumdahl4 Figure 4.5: When solid NaCl dissolves, the Na+ and Cl- ions are randomly dispersed in the water. Ions in solution conduct electricity. Figure 4.8: Acetic acid (HC2H3O2) exists in water mostly as undissociated molecules. Only a small percentage of the molecules are ionized. PDF Properties of solutions | Solubility and phase diagram • Liquids dissolved in water: the alcohol-water and the hydrogen peroxide-water systems are chosen, as examples of totally miscible liquids of very different applications: one is a 1. Salt water solutions phase diagram at 100 kPa (NaCl - H2O). E, eutectic point. Properties of some particular solutions. Why Does Water Dissolve Salt? | Chapter... | Middle School Chemistry Key Concepts. The polarity of water molecules enables water to dissolve many ionically bonded substances. Look at the teacher version of the activity sheet to find the questions and answers. Question to Investigate. How does salt dissolve in water? NaCl Material for Winter Maintenance and Its Environmental Effect The NaCl dissolved in storm water can be transferred in two ways. Aquifers play a role of reservoir wells for NaCl during winter [62], and become a source during summer, rejecting salted waters in the streams, thus contaminating surface waters [42, 54].
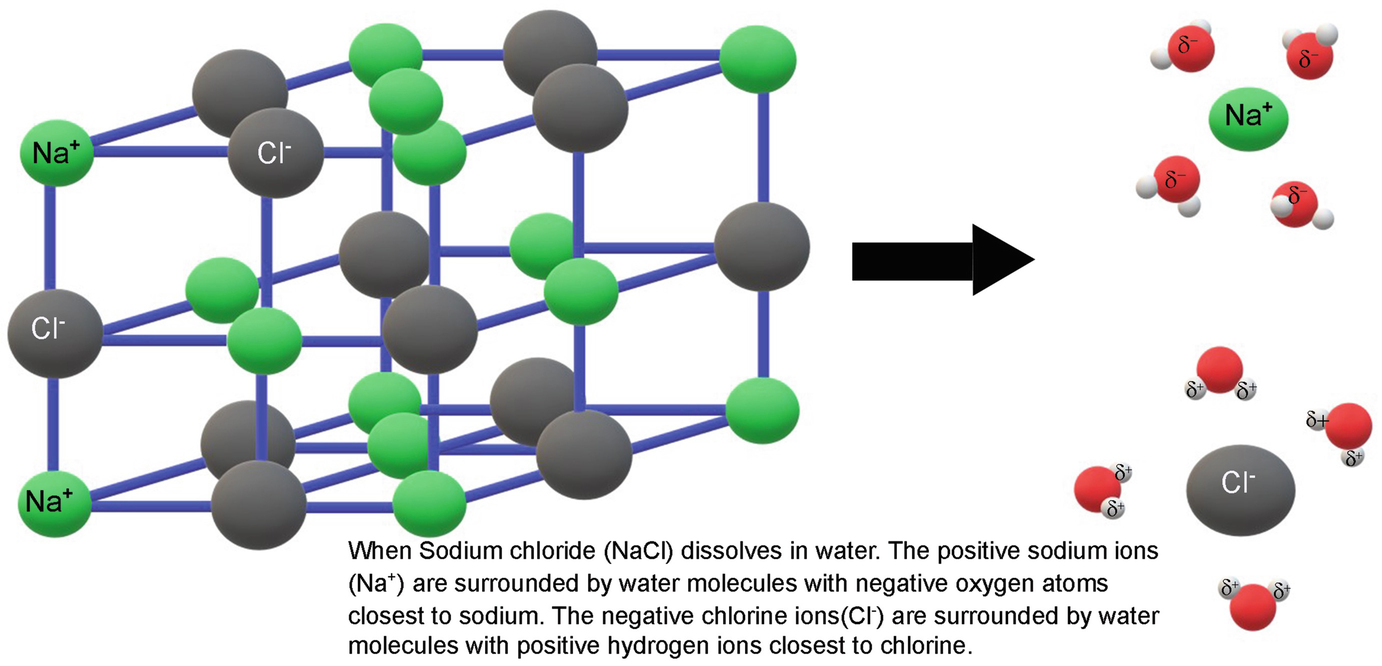
Solubility | Why Do Some Solids Dissolve in Water? When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these C12H22O11 molecules When one of these solids dissolves in water, the ions that form the solid are released into solution, where they become associated with the polar solvent molecules. Electronegativity: Why does sodium chloride (NaCl) dissolve in water... In conclusion, while sodium chloride (NaCl) dissolves in water due to the attractive forces with the polar water molecules overwhelming the forces between the positive sodium ions and the negative chloride ions, resulting in disassociation; silicon dioxide (SiO2) does not dissolve due to being a giant... What is the step by step process of dissolving of NaCl on water? The process by which NaCl dissolves in water is simple. Salt ions (Na+ and Cl-) on the corners, edges, and faces of NaCl crystals saturation with solid NaCl (halite) is about 6.1 molal in the diagram (pure water is about 0.998 g/ml). And just to confuse you: apparent molar volume is a measure of a... How does sodium chloride (NaCl) dissolve in water? Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na+) and chloride (Cl-) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three...
Dissolution of NaCl in Water - interactive simulations - eduMedia Sodium chloride (NaCl) is in fact the joining of an Na+ ion and a Cl- ion, which mutually attract one another via electrostatic attraction. This process continues until the salt is totally dissolved. Learning goals. To illustrate the different stages in the dissolution of salt (sodium chloride) in water.
How Does Sodium Chloride NaCl Dissolve in Water Stock Vector... Illustration about physics, energy, model, nacl, crystal, atomic, reaction, atom, lattice, chemistry, icon, ions, molecule - 184061480.
Sodium chloride - Wikipedia Phase diagram of water-NaCl mixture. The second major application of salt is for de-icing and anti-icing of roads, both in grit bins and spread by winter When dissolved in water, the sodium chloride framework disintegrates as the Na+ and Cl− ions become surrounded by polar water molecules.
Sodium chloride - New World Encyclopedia Sodium chloride, also known as common salt or table salt, is a chemical compound with the formula NaCl. Its mineral form is called halite. It is highly soluble in water and is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms.
concentration - 10 g of NaCl is dissolved in 250 g of water. To calculate molality, no. moles of $\ce{NaCl}$ is $10/58.5$. What does "percentage by mass" mean in the context of a compound dissolved in a solution?
Structural and electronic properties of NaCl dissolved in water @inproceedings{Gaiduk2014StructuralAE, title={Structural and electronic properties of NaCl dissolved in water}, author={Alex P Gaiduk and François Gygi and Giulia Galli}, year={2014} }.
How does table salt (NaCl) dissolve in water? A. The oxygen atoms... When placed in water, NaCl is surrounded first by water molecules and then NaCl dissociates to Na+ and Cl-. They are then attracted to the slightly negative (O) and positive (H) parts of water. Water does not really form 'hydrogen bond' with Na and Cl. It only forms hydrogen bond with compounds with...
Raman spectroscopic study of the effects of dissolved NaCl on water... For liquid water, a water molecule interacts with neighboring water molecules through various local hydrogen bonded networks. According to the simulations, the dissolved behaviors of NaCl in water are dependent on ion concentrations.
Draw a diagram of table salt (NaCl) dissolved in water. | Quizlet Find step-by-step Biology solutions and the answer to the textbook question Draw a diagram of table salt (NaCl) dissolved in water..
5.85g NaCl is dissolved in 500ml of water. The molarity of the solution... Dissolving 120 gm of a compound of (mol. wt. 60) in 1000 g of water gave a solution of density 1.5 g/mL. > 111.1g of the non-volatile solute, urea needs to be dissolved in 100 g of water, in order to decrease the vapour pressure of water > Memorization tricks. > Diagram set. > Problem solving tips.
Concentration Table 9.2 "Solubilities of Various Solutes in Water at 25°C (Except as Noted)" lists the solubilities of various solutes in water. A saline solution with a mass of 355 g has 36.5 g of NaCl dissolved in it. What is the mass/mass percent concentration of the solution?
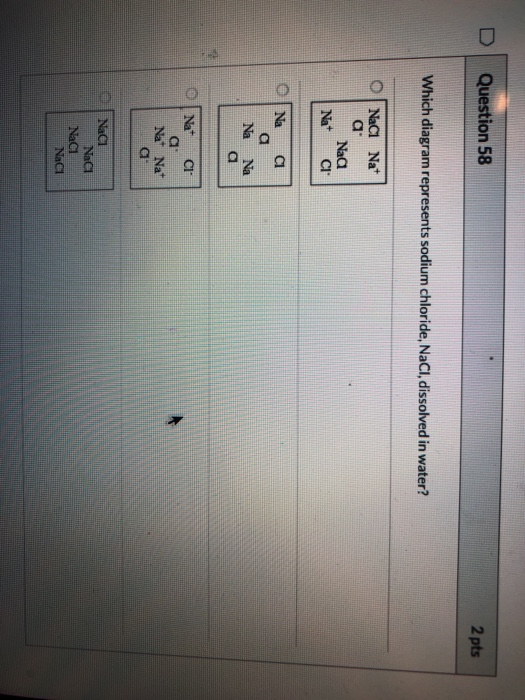
Solved Question 58 2 pts Which diagram represents... | Chegg.com Transcribed image text: Question 58 2 pts Which diagram represents sodium chloride, NaCl, dissolved in water?
What Happens When Salt Is Added to Water? | Sciencing "Salt dissolved in water" is a rough description of Earth's oceans. In chemistry, it results in a solution, as the ionic bond of NaCl is Reviewed by: Lana Bandoim, B.S. The sight of ordinary salt dissolved in water is, in all likelihood, entirely familiar to you, as the phenomenon literally dominates the globe.
Dissolving Salt in Water: Chemical or Physical Change? Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does...
Water's Solvent Properties | Introduction to Chemistry Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Dissociation of NaCl in waterWhen table salt (NaCl) is mixed in water, spheres of hydration form around the ions.














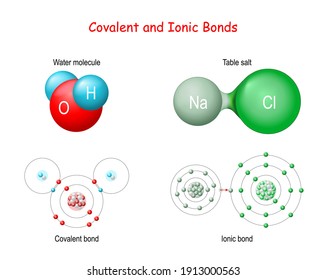
0 Response to "36 nacl dissolved in water diagram"
Post a Comment