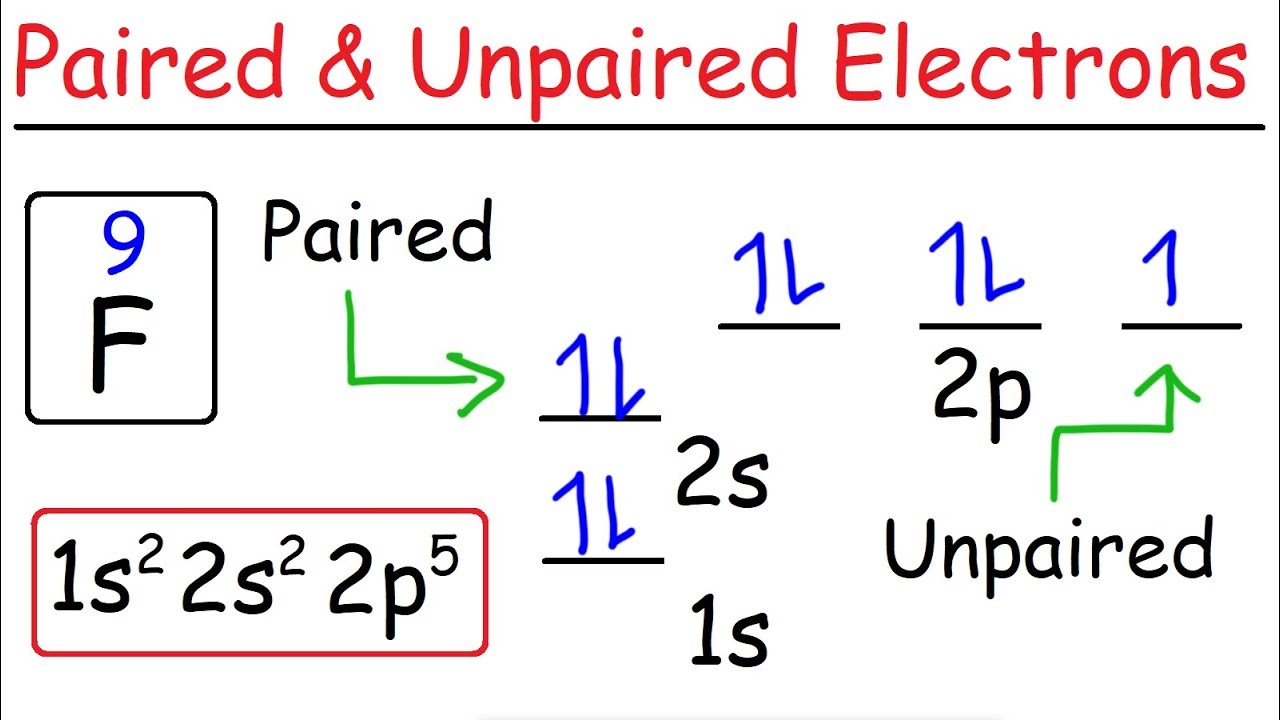
42 cr3+ orbital diagram
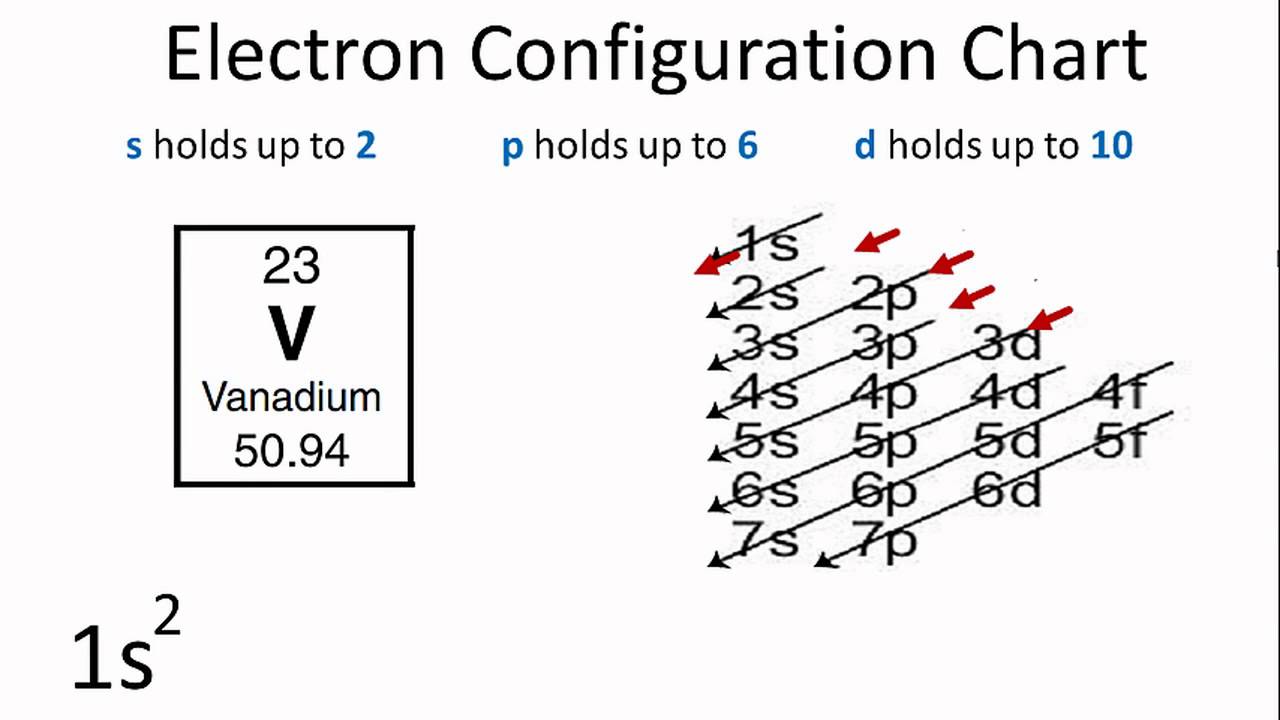
What is the orbital diagram for chromium? - Quora The figure below demonstrates the order in which the orbitals are filled by electrons. As you can see, the 4S orbital is filled BEFORE the 3D orbital as it has a lower energy, and therefore 3D has to be placed in the 4th row in the periodic table, after 4S. This trend continues as you go down the rows. Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
Write orbital diagram for Cr3+ | Chegg.com Question: Write orbital diagram for Cr3+ This question hasn't been solved yet Ask an expert Ask an expert Ask an expert done loading. Write orbital diagram for Cr 3+ Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
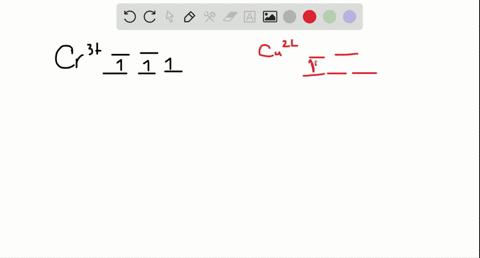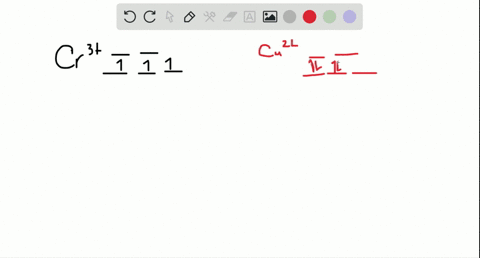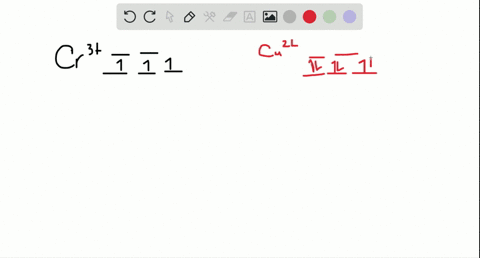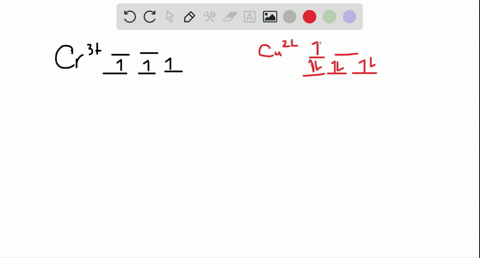
Cr3+ orbital diagram
Problem Set #4 (Ch 3, 4) Flashcards | Quizlet Specify the electron configurations for each of the following ions by developing the orbital diagram. The electron configuration will display below the diagram. O2-Br-Sr2+ Co3+ Cu2+ [He] 2s^2 2p^6 [Ar] 3d^10 4s^2 4p^6 [Ar] 3d^10 4s^2 4p^6 [Ar] 4s^0 3d^6 [Ar] 4s^0 3d^9. Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine ... Answered: 6) Using arrows to represent electrons,… | bartleby 6) Using arrows to represent electrons, Construct the orbital diagram of the Cr and Cr3+ ion. Cr Cr3+ Determine which one has the larger radius. (Select one answer) 7) O Ca Ca2+ OMg2+ Sr2+ 8) Amongst those four elements, which one does not follow the general electron affinity trend: (Select one answer) ON OF ఐ Question Need solution urgently Quimica Inorganica Descriptiva Rayner Canham - Academia.edu Si quieres tener buenas ideas, tienes que tener muchas ideas. La mayoría de ellas serán erróneas, y solo tienes que aprender cuáles desechar.
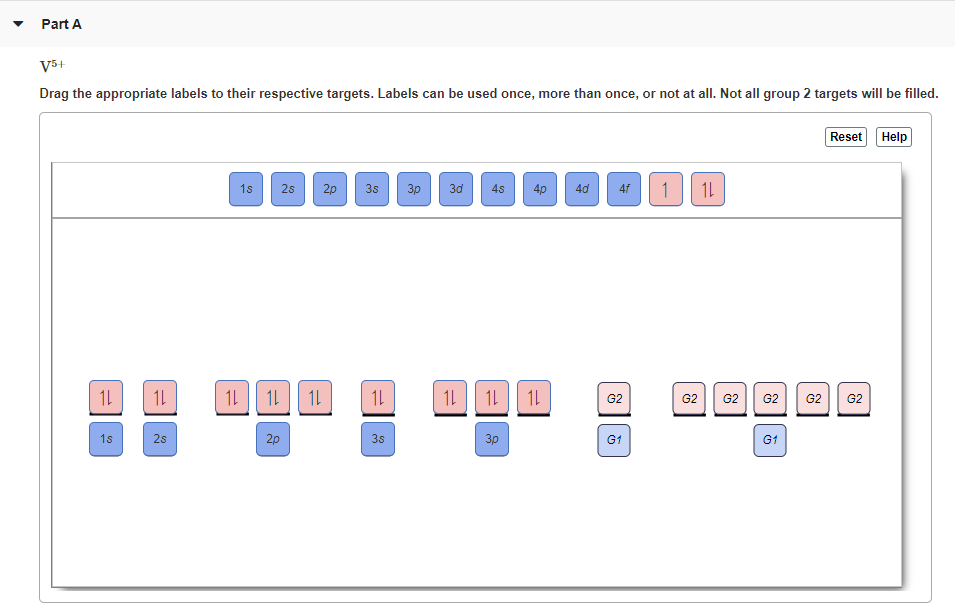
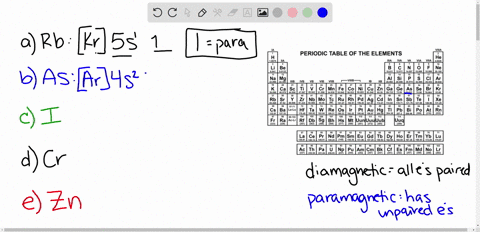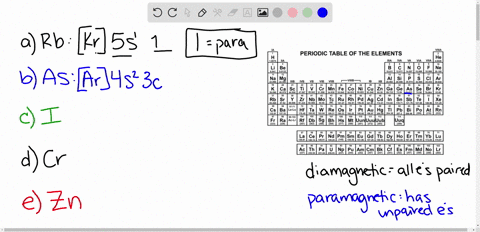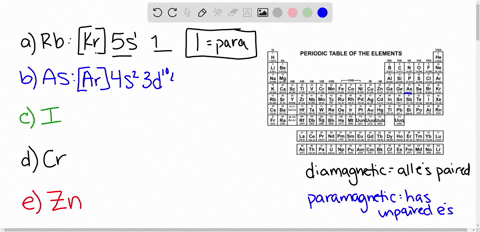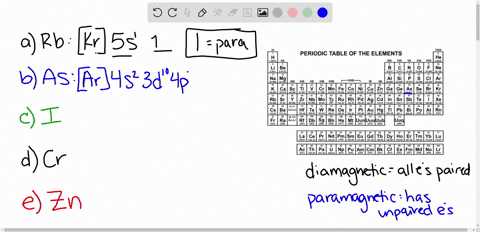
Cr3+ orbital diagram. Solved Part A Enter an orbital diagram for V5+ Drag the - Chegg Transcribed image text: Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled Reset Help P 111 1s 25 38 4s 2p 3p 4p 3d 61 G1 G1 GIG1 G1 GIG1 G1 G1 G1G1G1 G1|| G1 G16161 62 G2 G2 G2 G2 G2 G2 G2 Submit Request Answer Part B Enter an orbital diagram for Cr3 Drag the ... 1. Write orbital diagrams for each of these ions. *a. V5+ *b. Cr3+ *c ... Cr3+ C r 3 + c. N i2+ N i 2 + d. F e3+ F e 3 + Orbital Diagram Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons... What is the electron configuration of Cr 3+? Chemistry Q&A - Byju's Cr is an exception where the last electron enters into the 3d orbital instead of 4s orbital to attain half-filled stability. The electronic configuration of chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5. Cr 3 + is formed by losing three electrons from the neutral chromium atom. So, the electronic configuration of Cr 3 + is 1 s 2 2 s 2 2 ... Solved 42) Select the diamagnetic ion. Show the partial - Chegg Show the partial orbital diagram for each ion. A) Sc3+ B) Cr3+ C) Cu2+ D) Cr2+ E) Ni2+ Question: 42) Select the diamagnetic ion. Show the partial orbital diagram for each ion. A) Sc3+ B) Cr3+ C) Cu2+ D) Cr2+ E) Ni2+ This problem has been solved! See the answer See the answer See the answer done loading.
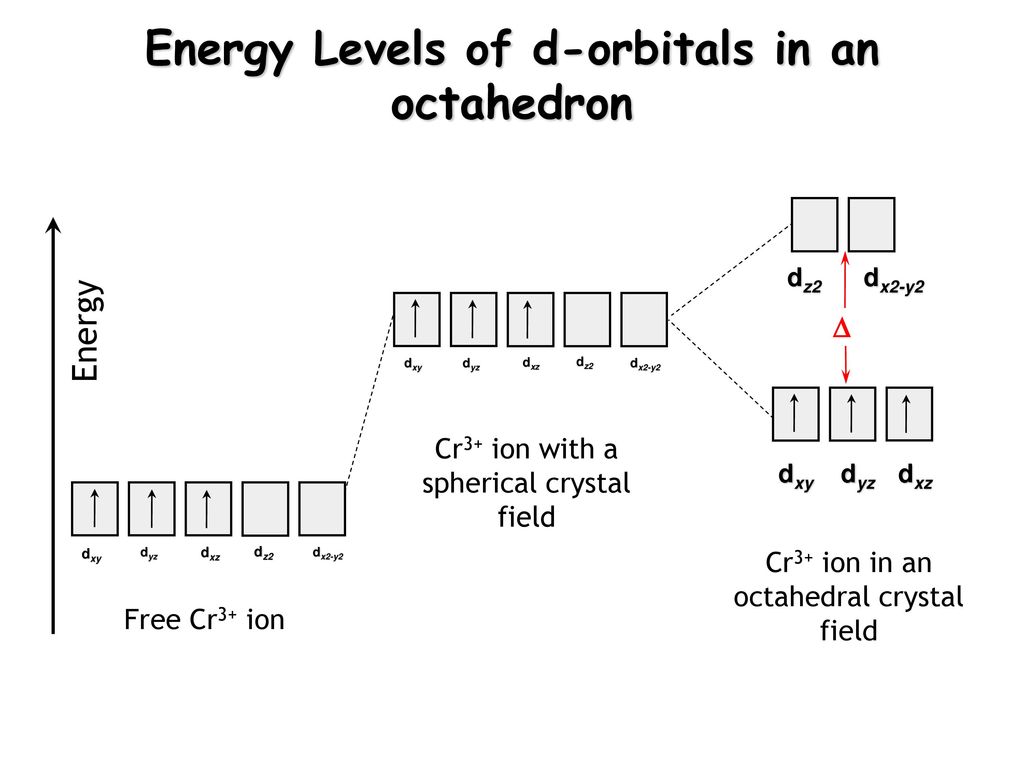
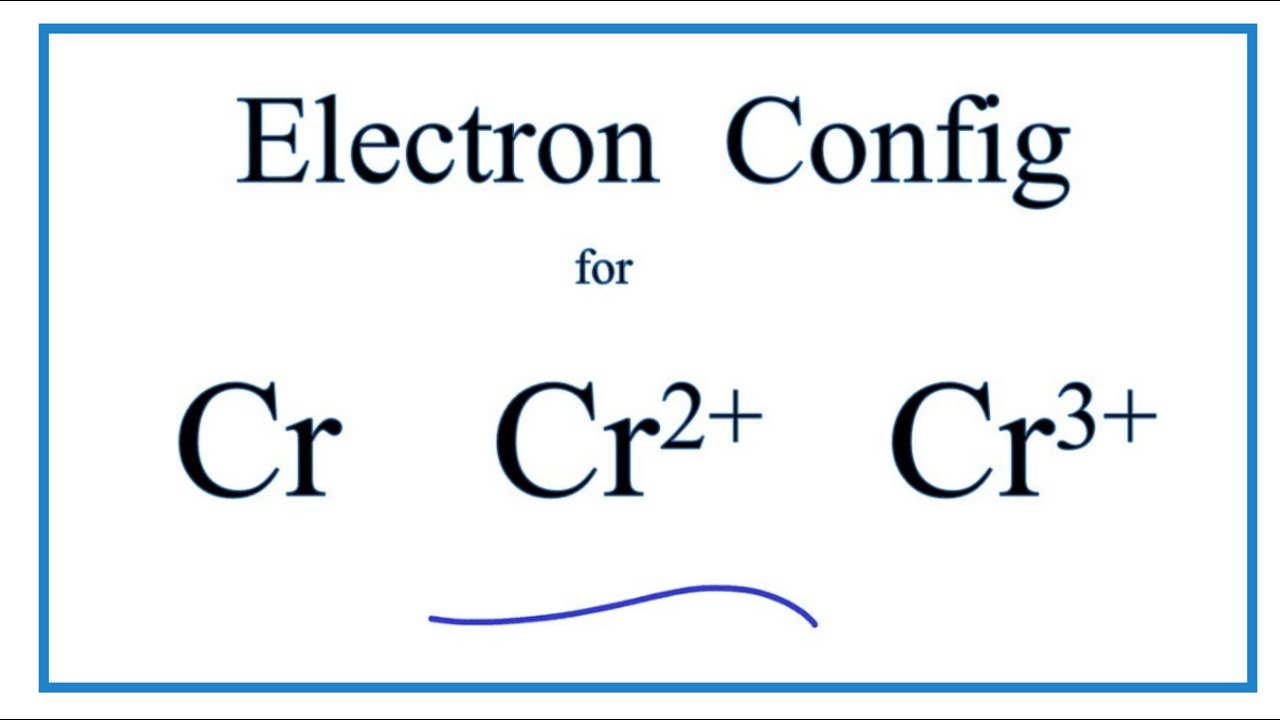
Absorption and Emission Spectroscopy of Cr3+ in ruby (Corundum) The optical absorption spectrum of corundum has broad band in the range of 421 to 429 nm and 555 to 560 nm, and the spin-forbidden transition in region about 700 nm. Studied ruby samples have two ... Electron Configuration for Chromium (Cr, Cr2+, Cr3+) Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chromium go in the 2s orbital. The next six electrons will go in the 2p orbital. SOLVED:Draw the octahedral crystal field splitting diagram ... - Numerade Draw the octahedral crystal field splitting diagram for each metal ion. a. Cr3+ b. Cu2+ c. Mn3+ (high- and low-spin) d. Fe2+ (low-spin) Answer. a) $\mathrm{Cr}^{3+}$ IMAGE NOT AVAILABLE ... Spin crystal filled orbital spitting diagram for em in Tripolis looks like and then for if it topless we has we have ah ah formal alike this hand in argon ... Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) We first need to find the number of electrons for the Cr atom (there are 24 electrons) using the Periodic Table. When we write the configuration, we'll put all 24 electrons in orbitals around the...
How to Write the Atomic Orbital Diagram for Chromium (Cr) To write the orbital diagram for the Chromium (Cr) first we need to write the electron configuration for just Cr. To do that we need to find the number of electrons for the Cr atom (there are 24... What is the electron configuration of Cr 3+? - Socratic.org The atomic number of Chromium is Z = 24, therefore a Cr atom possesses 24 electrons. Cr:1s22s22p63s23p64s13d5 Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Chromium(Cr) electron configuration and orbital diagram To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way. Quimica Inorganica Descriptiva Rayner Canham - Academia.edu Si quieres tener buenas ideas, tienes que tener muchas ideas. La mayoría de ellas serán erróneas, y solo tienes que aprender cuáles desechar.
Answered: 6) Using arrows to represent electrons,… | bartleby 6) Using arrows to represent electrons, Construct the orbital diagram of the Cr and Cr3+ ion. Cr Cr3+ Determine which one has the larger radius. (Select one answer) 7) O Ca Ca2+ OMg2+ Sr2+ 8) Amongst those four elements, which one does not follow the general electron affinity trend: (Select one answer) ON OF ఐ Question Need solution urgently
Problem Set #4 (Ch 3, 4) Flashcards | Quizlet Specify the electron configurations for each of the following ions by developing the orbital diagram. The electron configuration will display below the diagram. O2-Br-Sr2+ Co3+ Cu2+ [He] 2s^2 2p^6 [Ar] 3d^10 4s^2 4p^6 [Ar] 3d^10 4s^2 4p^6 [Ar] 4s^0 3d^6 [Ar] 4s^0 3d^9. Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine ...













![The degenerate orbitals of [ Cr (H2O)6 ]^3 + are:](https://haygot.s3.amazonaws.com/questions/1247765_1614169_ans_018cbb9a0874429f933e666797cca56c.PNG)









0 Response to "42 cr3+ orbital diagram"
Post a Comment