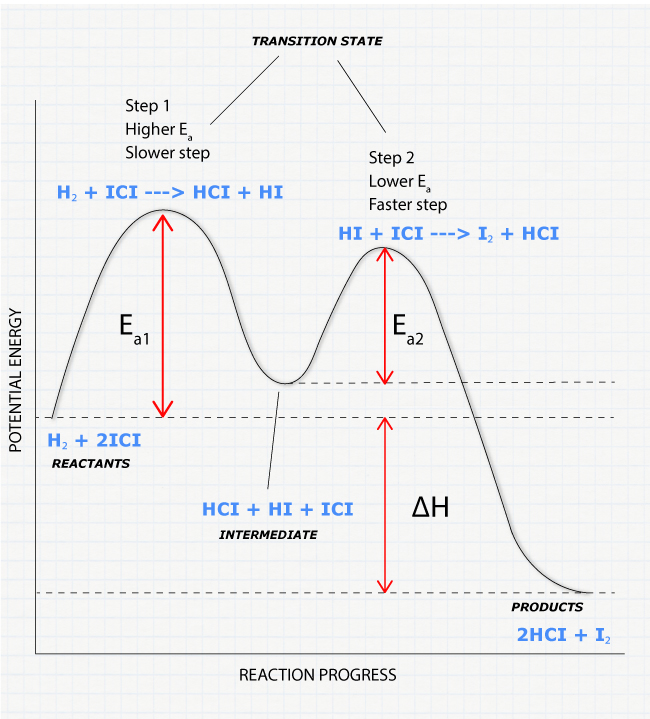
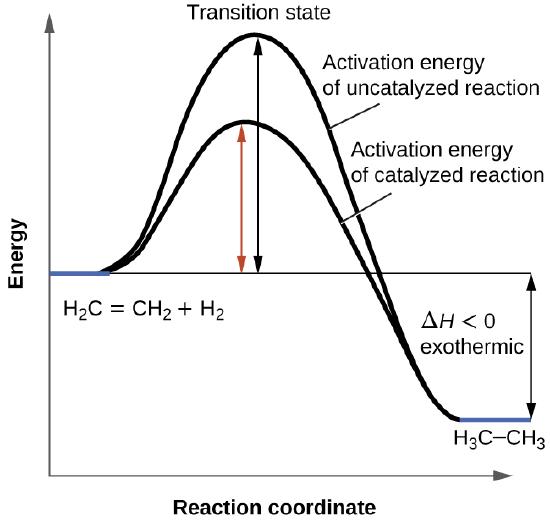
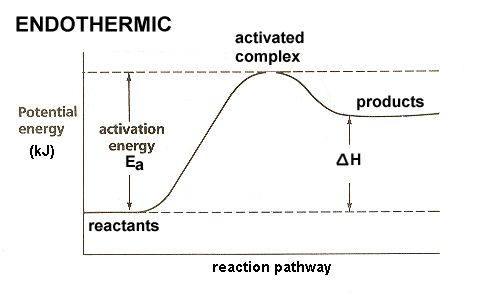
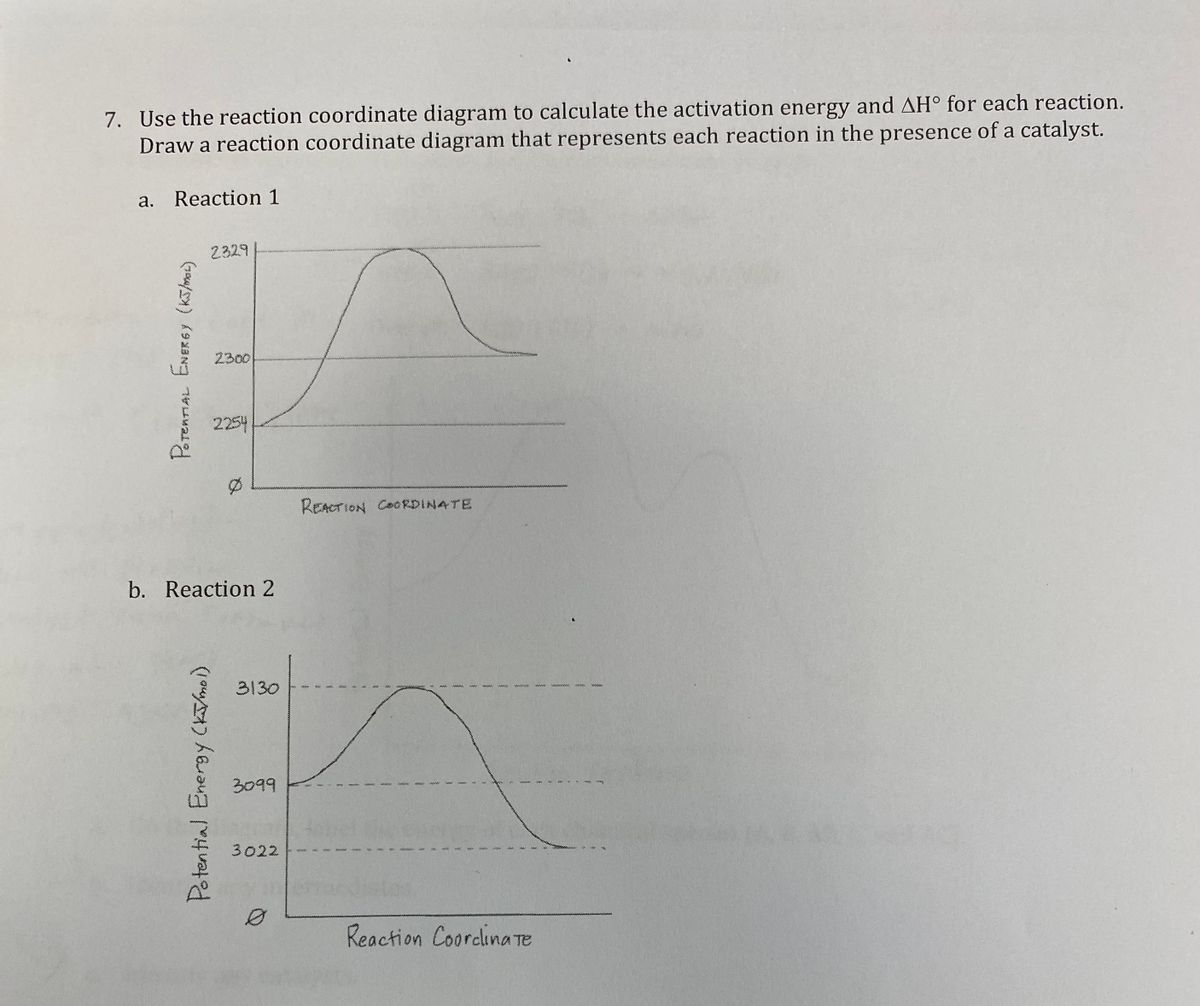
42 exothermic reaction coordinate diagram
› topics › engineeringArrhenius Equation - an overview | ScienceDirect Topics The Arrhenius equation (Arrhenius, 1889; see Chapter 1) for chemical kinetics was experimentally derived for aqueous solutions and electrolytic dissociation.It was known that the temperature T influences the reaction rate, expressed in terms of the so-called equilibrium rate constant κ = κ 1 /κ 2 representing the ratio between the individual rate constants κ 1 and κ 2 of the forward and ... › topics › chemistryCovalent Bond - an overview | ScienceDirect Topics Energy is released when the electrons associated with the two hydrogen atoms form a covalent bond. The process releases heat; therefore, it is exothermic.The heat released when one molecule of a compound forms at 298 K is the standard enthalpy change (ΔH°) for the process.
› topics › chemicalHydrotreating - an overview | ScienceDirect Topics Hydrotreating is a well-established and industrially acceptable process to refine the crude petroleum and production of transportation fuels. In this process, a high volume of hydrogen gas is used for the removal of the undesired impurities (e.g., sulfur, nitrogen, oxygen, etc.) in crude petroleum to reduce the emission of pollutant gases (e.g., SOx and NOx) during its consumption, and also ...
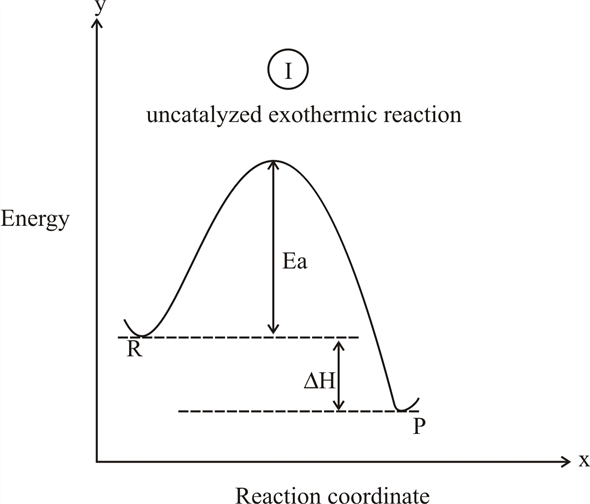
Exothermic reaction coordinate diagram
en.wikipedia.org › wiki › Reaction_rateReaction rate - Wikipedia Each reaction rate coefficient k has a temperature dependency, which is usually given by the Arrhenius equation: =. E a is the activation energy; R is the gas constant.Since at temperature T the molecules have energies given by a Boltzmann distribution, one can expect the number of collisions with energy greater than E a to be proportional to e −E a ⁄ RT. en.wikipedia.org › wiki › Stability_constants_ofStability constants of complexes - Wikipedia The first step is then a substitution reaction involving the displacement of a bound water molecule by ammonia forming the tetrahedral complex [Ag(NH 3)(H 2 O) 3] +. In the second step, all the aqua ligands are lost and a linear, two-coordinate product [H 3 N–Ag–NH 3] + is formed. chem.libretexts.org › Bookshelves › Physical_andBond Energies - Chemistry LibreTexts Aug 21, 2020 · In some reactions, the energy of the products is lower than the energy of the reactants. Thus, in the course of the reaction, the substances lose energy to the surrounding environment. Such reactions are exothermic and can be represented by an energy-level diagram in Figure 1 (left). In most cases, the energy is given off as heat (although a ...
Exothermic reaction coordinate diagram. › team › statsGiants Team | New York Giants – Giants.com New York Giants Team: The official source of the latest Giants roster, coaches, front office, transactions, Giants injury report, and Giants depth chart chem.libretexts.org › Bookshelves › Physical_andBond Energies - Chemistry LibreTexts Aug 21, 2020 · In some reactions, the energy of the products is lower than the energy of the reactants. Thus, in the course of the reaction, the substances lose energy to the surrounding environment. Such reactions are exothermic and can be represented by an energy-level diagram in Figure 1 (left). In most cases, the energy is given off as heat (although a ... en.wikipedia.org › wiki › Stability_constants_ofStability constants of complexes - Wikipedia The first step is then a substitution reaction involving the displacement of a bound water molecule by ammonia forming the tetrahedral complex [Ag(NH 3)(H 2 O) 3] +. In the second step, all the aqua ligands are lost and a linear, two-coordinate product [H 3 N–Ag–NH 3] + is formed. en.wikipedia.org › wiki › Reaction_rateReaction rate - Wikipedia Each reaction rate coefficient k has a temperature dependency, which is usually given by the Arrhenius equation: =. E a is the activation energy; R is the gas constant.Since at temperature T the molecules have energies given by a Boltzmann distribution, one can expect the number of collisions with energy greater than E a to be proportional to e −E a ⁄ RT.

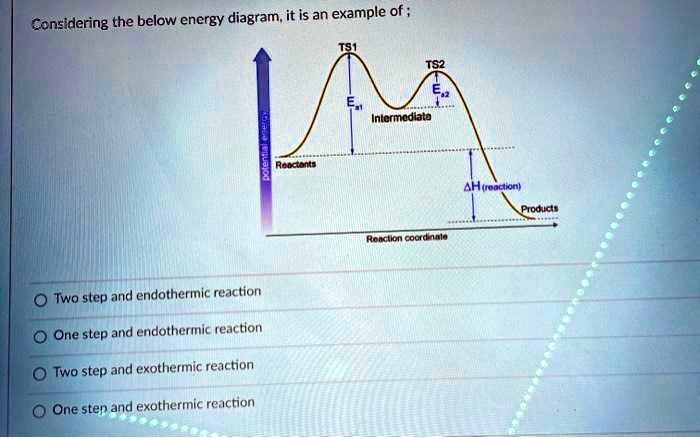
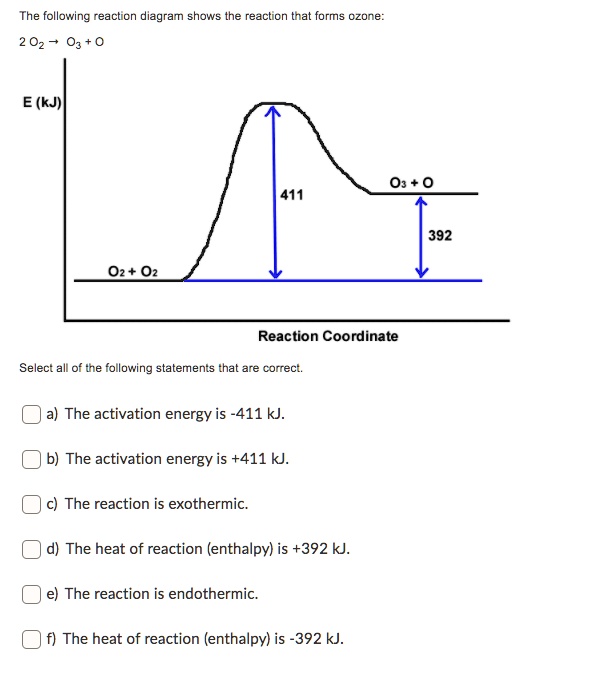





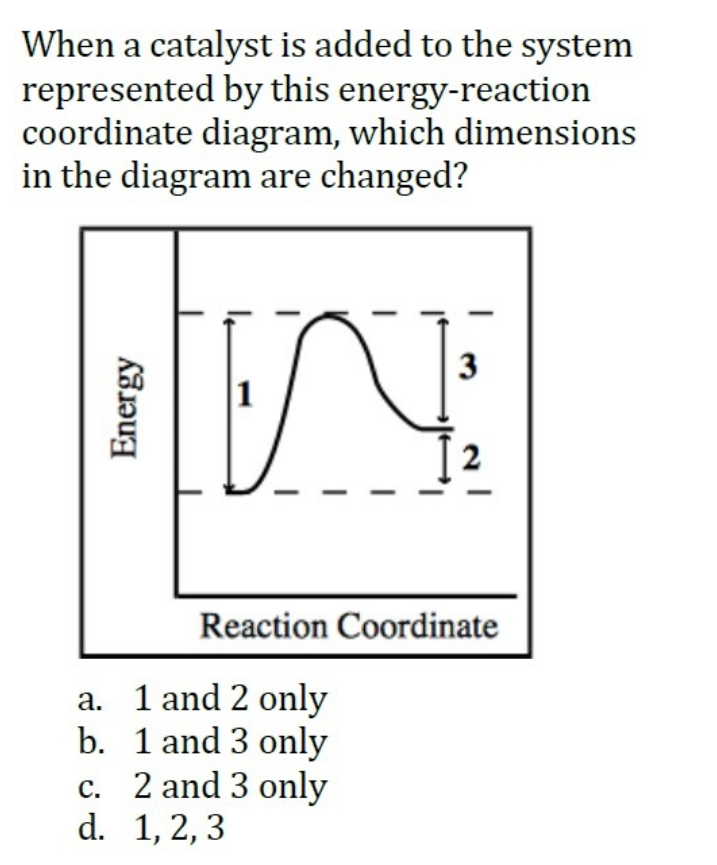

















0 Response to "42 exothermic reaction coordinate diagram"
Post a Comment