45 lewis diagram for hcn
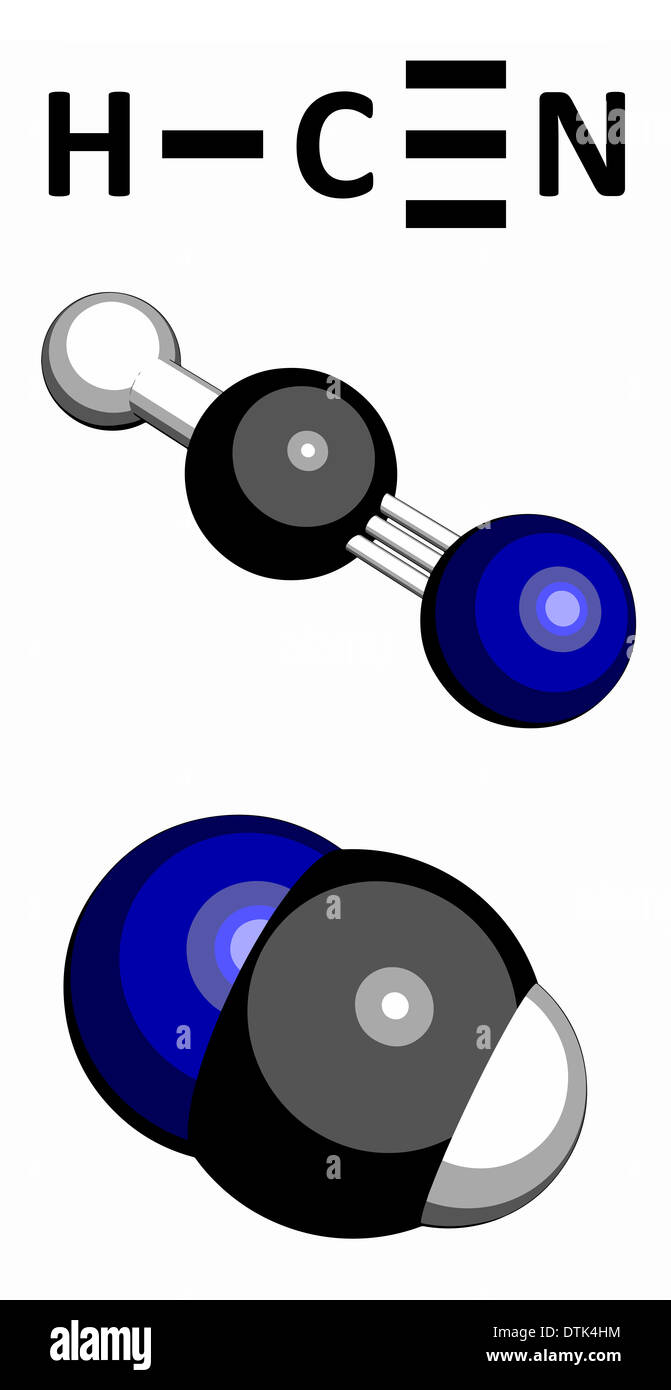
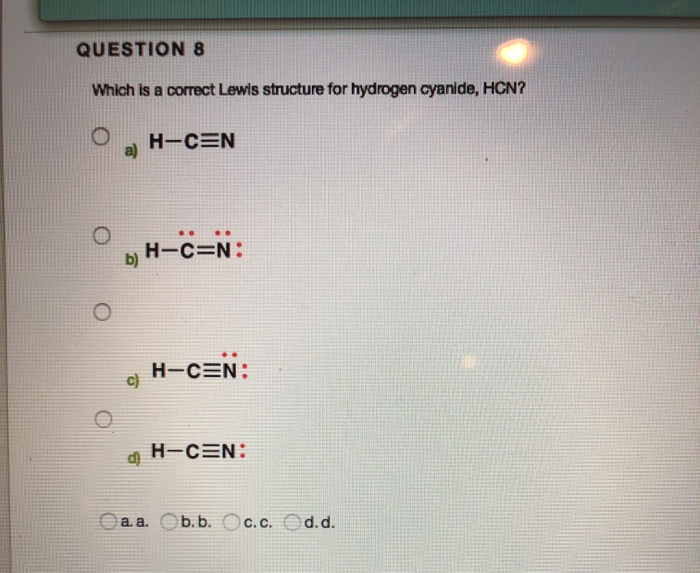
Lewis structure of HCN? - Answers What is the Lewis structure for HCN? H:C:::N:The Lewis structure for HCN, otherwise known as hydrogen cyanide, is fairly simple. Place the carbon atom in the center and triple bond it to a ... HCN Lewis structure, Molecular geometry, Hybridization, Polar or ... Steps for drawing Lewis dot structure of HCN Step 1. Count the total number of valence electrons present on each atom of the HCN compound. The valence electrons of all three atoms i.e hydrogen, carbon, and nitrogen, must be determined here to find the valance electrons of HCN.
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and ... HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity Hydrogen Cyanide is a very toxic acid and is famous for causing irritation in the eyes and respiratory system if any human inhales HCN in substantial quantity. The compound is a colorless substance that is available in liquid or gaseous form.
Lewis diagram for hcn
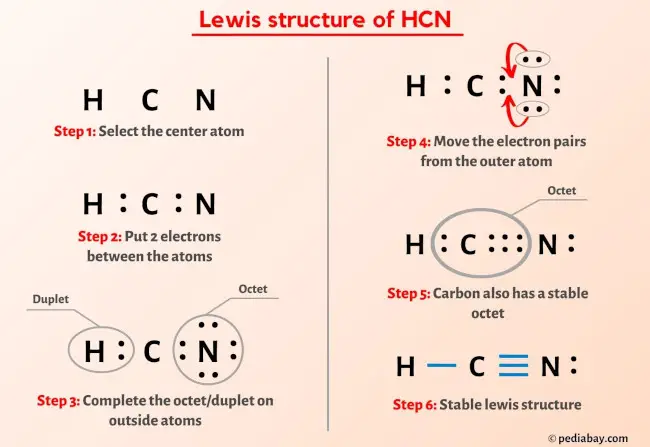
HCN Lewis Structure (Hydrogen Cyanide) - YouTube Hey Guys !In this video we will look at the Lewis Structure of Hydrogen Cyanide having a chemical formula of HCN. The molecule is made up of one Hydrogen ato... HCN Lewis Structure in 6 Steps (With Images) - Pediabay HCN lewis structure has a Carbon atom (C) at the center which is surrounded by one Hydrogen atom (H) and one Oxygen atom (O). There is a triple bond between the Carbon (C) & Nitrogen (N) atom and a single bond between Carbon (C) & Hydrogen (H) atom. There is 1 lone pair on the Nitrogen atom (N). What is the Lewis structure of HCN class 11 chemistry CBSE - Vedantu HCN, which is described by the chemical formula, is one of those molecules with a unique Lewis structure. Electroplating, refining, and as a base for other substances all use this liquid. Complete answer: It is essential to know the total number of valence electrons in any molecule before drawing the Lewis dot structure.
Lewis diagram for hcn. Unit 11: Lewis Structures Diagram | Quizlet Unit 11: Lewis Structures. Flashcards. Learn. Test. Match. Flashcards. Learn. Test. Match. Created by. Foxhollow2002. Unit Review Skyridge Chemistry - CJ Reid @i_luvpenguinz. Terms in this set (33) Which is the lewis diagram for H₂O?... Which is the molecular shape of H₂O?... Lewis structure calculator | Lewis structure generator To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO 4 – 2, we must first enter the charge by typing (-2) or by entering -2 in the charge field and pressing the «Add» button. Then we write the rest of the formula being as follows: (-2)SO4. Answered: Compound X has a molar mass of 579.36… | bartleby Sep 25, 2022 · Q: 5 Identify the acid-base conjugate pairs in each of the following reactions: (a) CH₂COO + HCN (b)… A: Given Chemical reactions Conjugate acid-base concept: A conjugate acid-base pair consists of two… Lewis Structure and Hybridization of HCN (hydrocyanic ... - ChemistNate Lewis Structure of HCN Watch on What is the hybridization of Carbon in HCN? Carbon is triple-bonded to nitrogen, and so there are two pi bonds (Remember: The first bond between any two atoms is a sigma bond, and the second/third bonds are pi bonds). This means two p orbitals are required to be left over after hybridization.
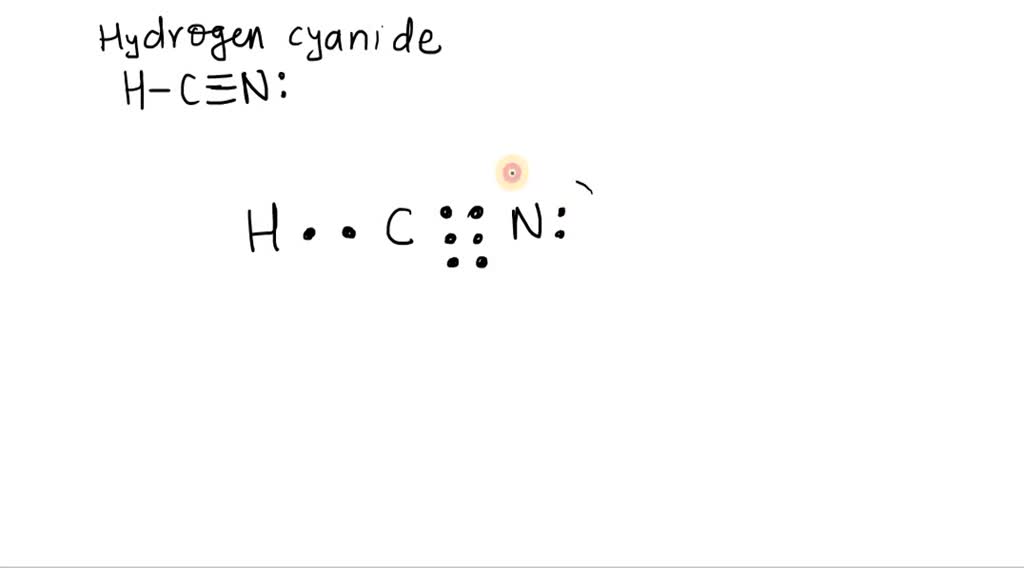
Introduction to ORGANIC CHEMISTRY MECHANISMS, dictionary … Electrophiles are Lewis acids - electron pair acceptors. e.g. organic reaction examples include Br +, CH 3 +, or the polar H δ +-Br δ- and H δ +-O δ-SO 2 OH (H 2 SO 4) Lewis base: An atom, ion or molecule that can donate a pair of electrons to form a bond. Nucleophiles are Lewis bases - electron pair donors. HCN Lewis Dot Structure: Drawing And Detailed Explanations To determine the HCN Lewis Dot Structure first we need to look for valence electrons in individual atoms. Hydrogen (Atomic number = 1 and electronic configuration = 1) belongs to the 1 st group of the periodic table and consists of only 1 electron. Lewis Structure of HCN - Root Memory Lewis Structure of HCN The lewis structure of HCN contains one single bond and one triple bond, with carbon in the center, and hydrogen and nitrogen on either side. There is one lone pair on the nitrogen atom, and hydrogen atom and carbon atom do not have any lone pair. How to Draw Lewis Structure of HCN? How to draw Lewis structure of HCN class 11 chemistry CBSE - Vedantu Complete answer: Lewis structure basically tells us how the electrons are paired. In this every dot represents an electron and pair of dots between chemical symbols for atoms represents the bond. The main steps of draw a Lewis structure are as follows: 1. Calculate the valence electrons present in the molecule this can be calculated by adding ...
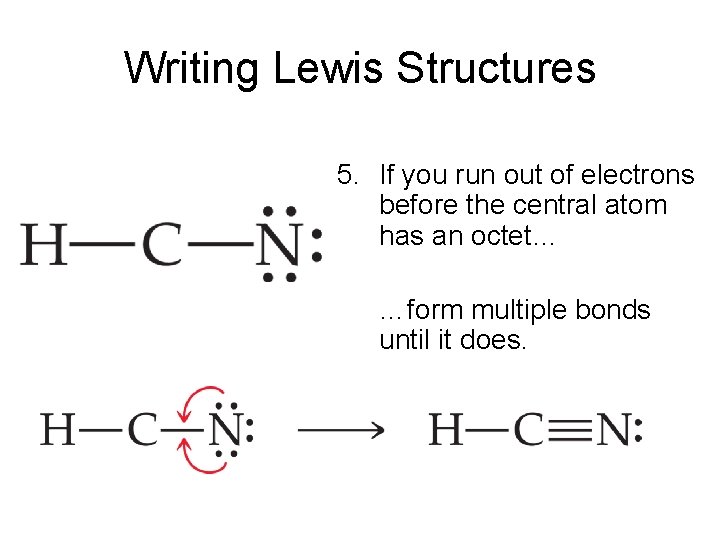
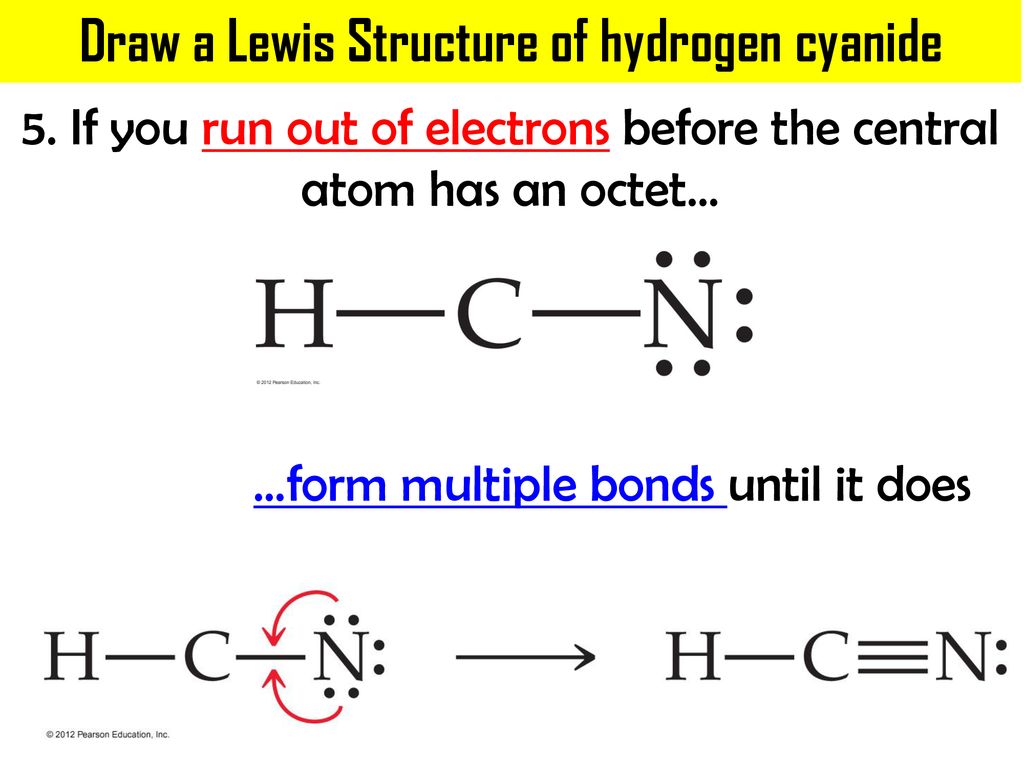
Chemical Bonding: HCN Lewis Structure - The Geoexchange We have a total of ten valence electrons for the HCN Lewis structure. We'll put two between atoms to form chemical bonds, so we've used four, then we'll go around the Nitrogen, six, eight, and ten. So when we look at the Lewis structure, Nitrogen had eight valence electrons, but the Carbon only has four. Lewis Structure of HCN (With 6 Simple Steps to Draw!) - Knords Learning Lewis structure of HCN contains a single bond between a Carbon (C) & Hydrogen (H) atom and a triple bond between the Carbon (C) and Nitrogen (N) atom. The Carbon atom (C) is at the center and it is surrounded by Hydrogen (H) and Nitrogen atom (N). The Nitrogen atom has 1 lone pair. Let's draw and understand this lewis dot structure step by step. What is the Lewis structure for HCN? - Answers Best Answer. Copy. H:C:::N: The Lewis structure for HCN, otherwise known as hydrogen cyanide, is fairly simple. Place the carbon atom in the center and triple bond it to a nitrogen atom. Then bond ... I need help with lewis diagram of HCN - which one do I put in the ... The Lewis dot diagram for hydrogen cyanide, #"HCN"#, is #"H:C:::N:"#.There is a single covalent bond between the hydrogen and carbon atom, represented by two dots, #:#, each of which represents a shared electron; a triple covalent bond between the carbon and nitrogen atom, represented by three pairs of dots, #:::#, representing three pairs of shared electrons, and a lone pair of electrons on ...
JEE Main 2023, 2024 Syllabus [Updated] - Download PDF Bronsted - Lowry and Lewis) and their ionization, acid-base equilibria (including multistage ionization) and ionization constants, ionization of water. pH scale, common ion effect, hydrolysis of salts and pH of their solutions, the solubility of sparingly soluble salts and solubility products, buffer solutions. Redox reactions and electrochemistry
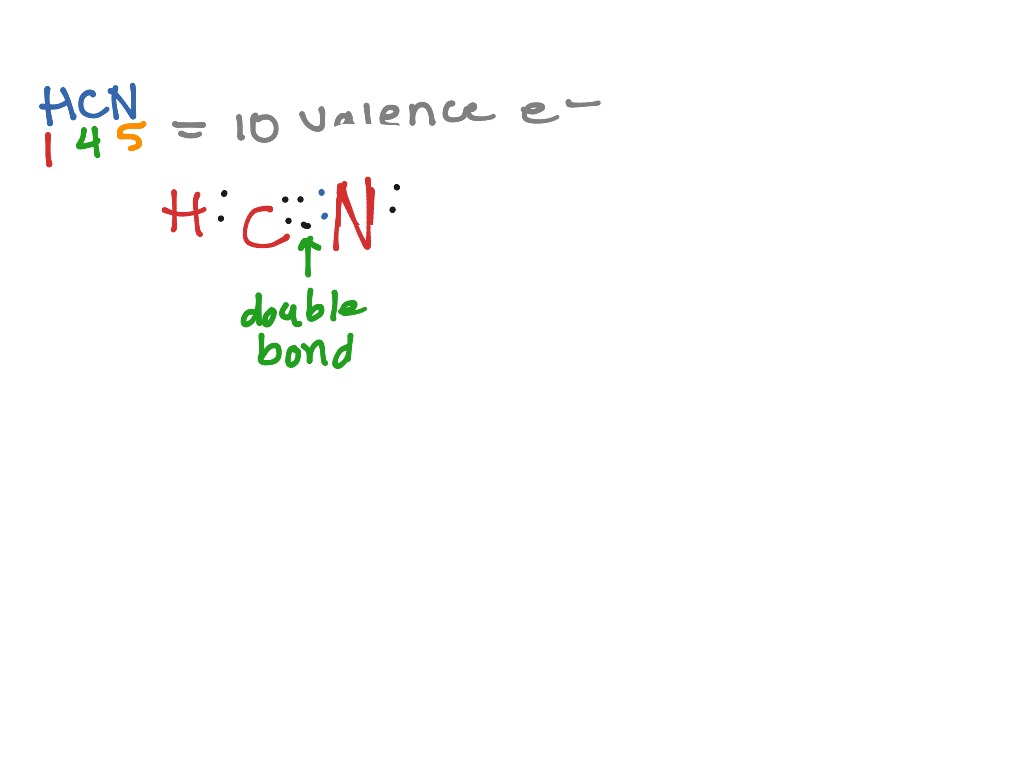
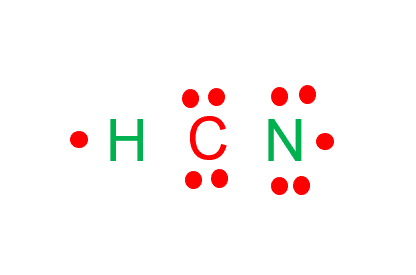
How to draw HCN Lewis Structure? - Science Education and Tutorials It is represented by dots in the HCN Lewis diagram. The HCN molecule's core carbon atom can be represented as follows: Total outermost valence shell electron of carbon atom in HCN= 4 Total outermost valence shell electron of nitrogen atom in HCN= 5 Total outermost valence shell electron of hydrogen atom in HCN= 1
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO ... CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN).
Chemistry Article - Topblogtenz Gold Bohr Model – How to draw Bohr diagram for Gold (Au)? Vishal Goyal. Krypton Bohr Model – How to draw Bohr diagram for Krypton(Kr) Vishal Goyal. Iodine Bohr Model – How to draw Bohr diagram for Iodine(I) Vishal Goyal. Copper Bohr Model – How to draw Bohr diagram for Copper(Cu) Vishal Goyal. Iron Bohr Model – How to draw Bohr diagram for Iron(Fe) Vishal …
HCN Lewis structure, Molecular geometry, Bond angle, Shape The total valence electrons available for drawing the HCN Lewis structure are 10. The HCN molecule has an identical electron and molecular geometry or shape i.e., linear. The C and N atoms present in the HCN molecule are sp hybridized. The HCN atoms form a mutual bond angle of 180° due to the molecule's linear shape.
What is the Lewis structure of HCN? | Homework.Study.com The Lewis dot structure for HCN is: This can be arrived at by following 5 general steps for writing Lewis dot structures. 1. Determine the number of valence electrons for each atom. The valence...
Endothelium - Wikipedia The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels, and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. Endothelial cells form the barrier between vessels and tissue and control the flow of substances and fluid into and out of a tissue.
How to draw Lewis Structure for HCN? - toppr.com Answer Here's how to do it. Explanation: Step 1. Draw a skeleton structure Put the least electronegative atom C in the middle with H and Cl on either side. H-C-N tep 2. Count the valence electrons you can use H + C + N =1 + 4 + 5 = 10 Step 3. Add these electrons to give every atom an octet You nave to put a triple bond between C and N.
HCN Lewis Structure, Molecular Geometry, Shape, and Polarity To start with making the Lewis Structure of HCN, we will first determine the central atom. And then place the remaining atoms in the structure. As Carbon is the least electronegative atom in this molecule, it will take the central position. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this:
HCN(Hydrogen Cyanide); Lewis Structure, Molecular Geometry ... Draw the Lewis Structure of HCN in 3 Easy Steps The Lewis structure merely takes into account the electrons present in the valence shell, neglecting the inner shell's electrons. A dot represents a lone pair of electrons in a Lewis structure, whereas a straight line indicates a bond pair of electrons. Step # 1
Chromic acid - Wikipedia The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide.This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, H 2 CrO 4 of which the trioxide is the anhydride.
Lewis Structure of HCN - YouTube The Lewis Structure (Lewis Dot Diagram) for HCN.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out...
What is the Lewis structure of HCN? | Socratic The carbon atom has (or shares) 3 electrons from the triple bond, and a lone pair of electrons, which it owns. With 2 inner core electrons, this makes 7 electrons with which it is associated. Since, the atomic number of carbon is 6, the carbon atom is formally negatively charged. When I write H −C ≡ N:, the carbon atom has a share of 4 ...
HCN Hybridization: Drawing, Structure And Detailed Explanations Lewis structure or lewis dot structure helps to figure out the valance electrons or hybridization of any compound. To determine the lewis structure of HCN, valance electrons of carbon, nitrogen and hydrogen should be counted from their electron configuration. Valance electron of hydrogen, carbon and nitrogen are 1, 4 and 5 respectively.
HCN Lewis Structure - Learnool HCN (hydrogen cyanide) has one hydrogen atom, one carbon atom, and one nitrogen atom. In the lewis structure of HCN, there is a single bond between carbon and hydrogen atom, and a triple bond between carbon and nitrogen atom, and on nitrogen atom, there is one lone pair. Steps Here's how you can draw the HCN lewis structure step by step.
Lewis Dot Diagram Of Hcn An example of linear electron pair and molecular geometry is BeH2. In this example, HCN, the Lewis diagram shows carbon at the center. It is determined by the fact that carbon makes four bonds with four valence electrons, nitrogen makes five bonds with five valence electrons and. Answer to Draw the Lewis structure for HCN. Include lone pairs.
Lewis Structure of H2O (With 6 Simple Steps to Draw!) - Knords Learning Step #1: Calculate the total number of valence electrons. Here, the given molecule is H2O (water). In order to draw the lewis structure of H2O, first of all you have to find the total number of valence electrons present in the H2O molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
HCN Lewis Structure & Molecular Geometry - What's Insight Hydrogen Cyanide (HCN) is a colorless, flammable, and poisonous liquid. The HCN Lewis structure comprises three different atoms: hydrogen, carbon, and nitrogen. It is a polar molecule with a bond angle of 180 degrees. HCN is used in electroplating, mining, and as a precursor for several compounds. Name of molecule.
What is the Lewis structure of HCN class 11 chemistry CBSE - Vedantu HCN, which is described by the chemical formula, is one of those molecules with a unique Lewis structure. Electroplating, refining, and as a base for other substances all use this liquid. Complete answer: It is essential to know the total number of valence electrons in any molecule before drawing the Lewis dot structure.
HCN Lewis Structure in 6 Steps (With Images) - Pediabay HCN lewis structure has a Carbon atom (C) at the center which is surrounded by one Hydrogen atom (H) and one Oxygen atom (O). There is a triple bond between the Carbon (C) & Nitrogen (N) atom and a single bond between Carbon (C) & Hydrogen (H) atom. There is 1 lone pair on the Nitrogen atom (N).
HCN Lewis Structure (Hydrogen Cyanide) - YouTube Hey Guys !In this video we will look at the Lewis Structure of Hydrogen Cyanide having a chemical formula of HCN. The molecule is made up of one Hydrogen ato...


![CHEMISTRY TUTORIAL] Lewis Structure of Hydrogen Cyanide ...](https://images.squarespace-cdn.com/content/v1/5f02d28f35d64d2a5022eeb1/1604451040567-7LIIA50HAGVJU0SH608N/Lewis+Structures+HCN.png)



















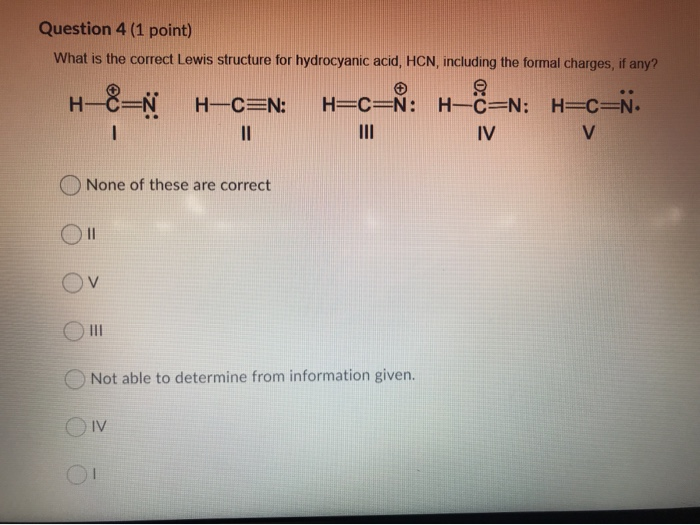



0 Response to "45 lewis diagram for hcn"
Post a Comment