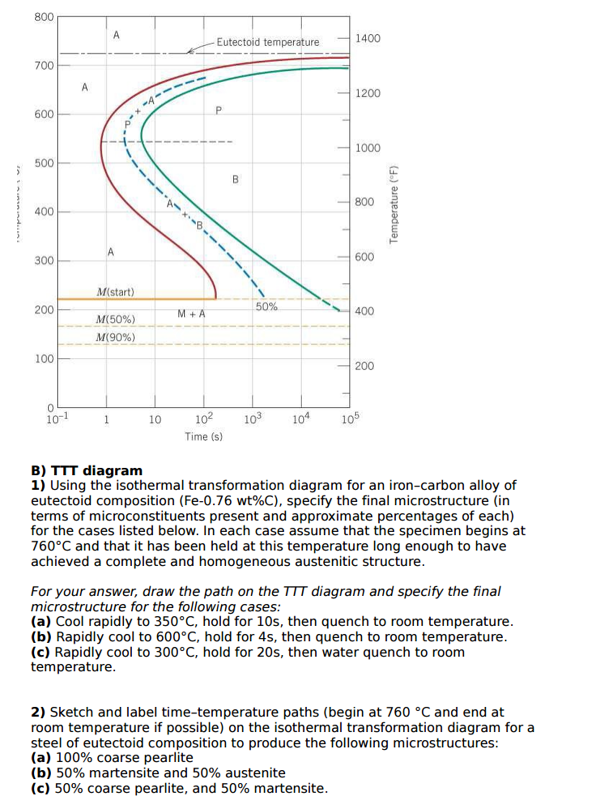
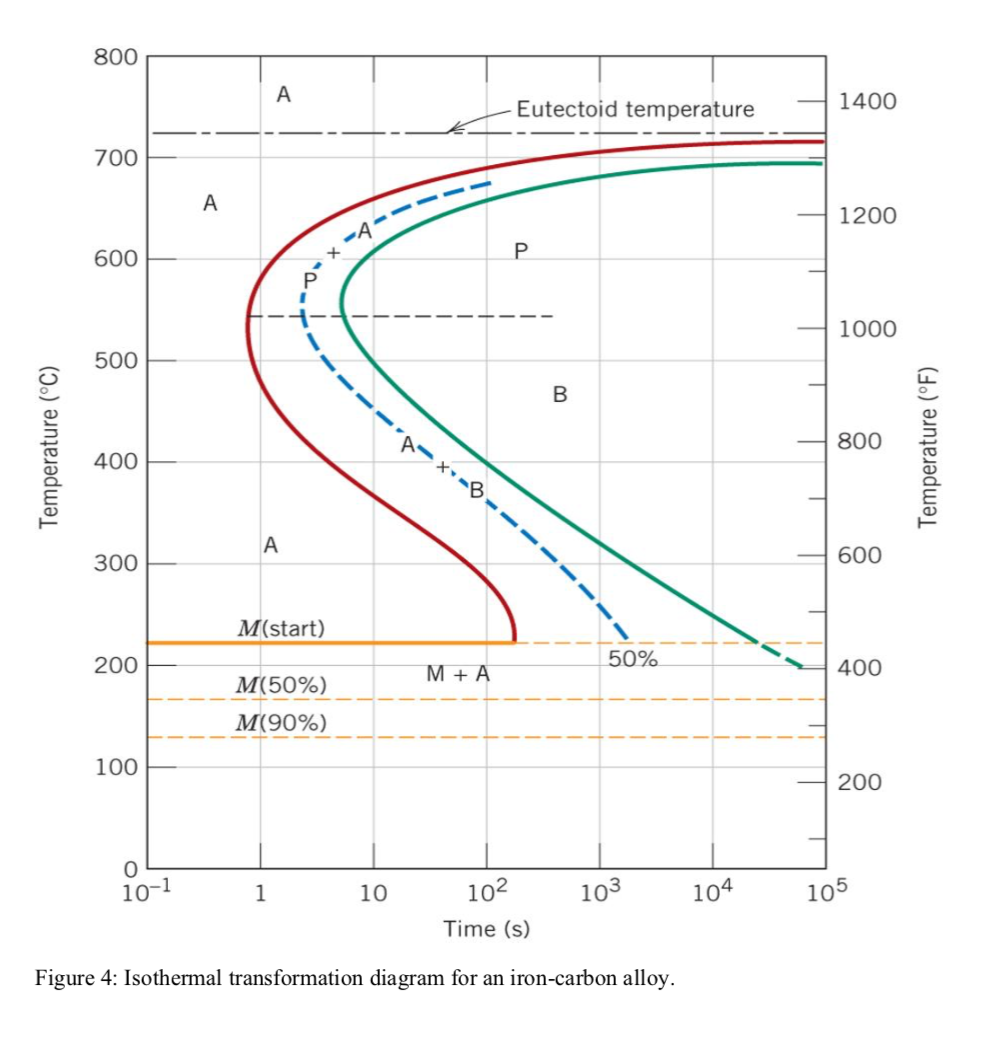
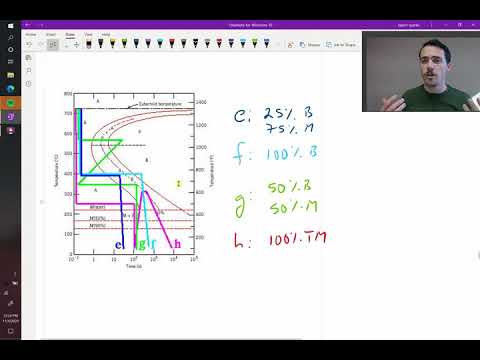
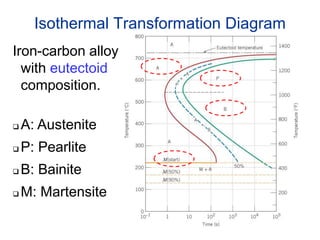
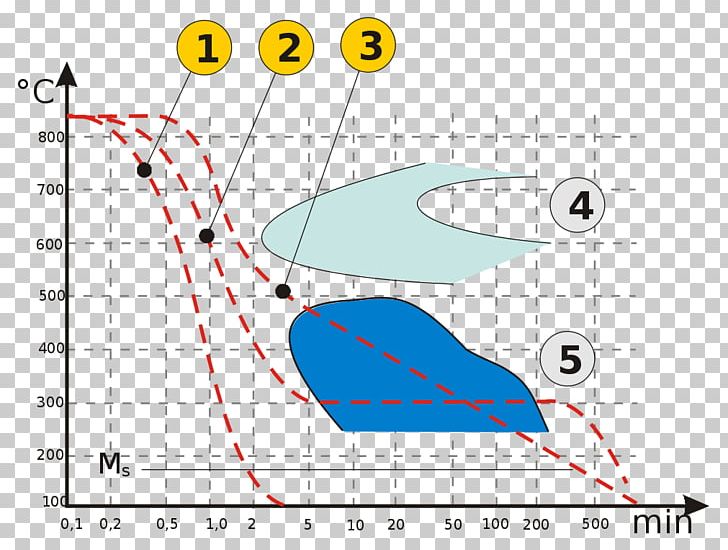
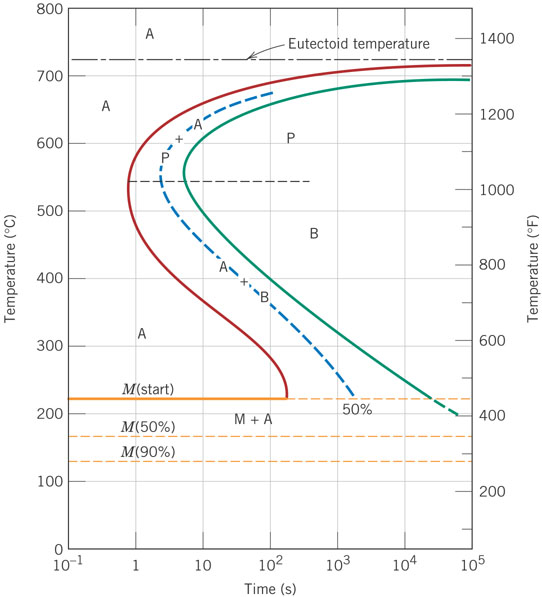
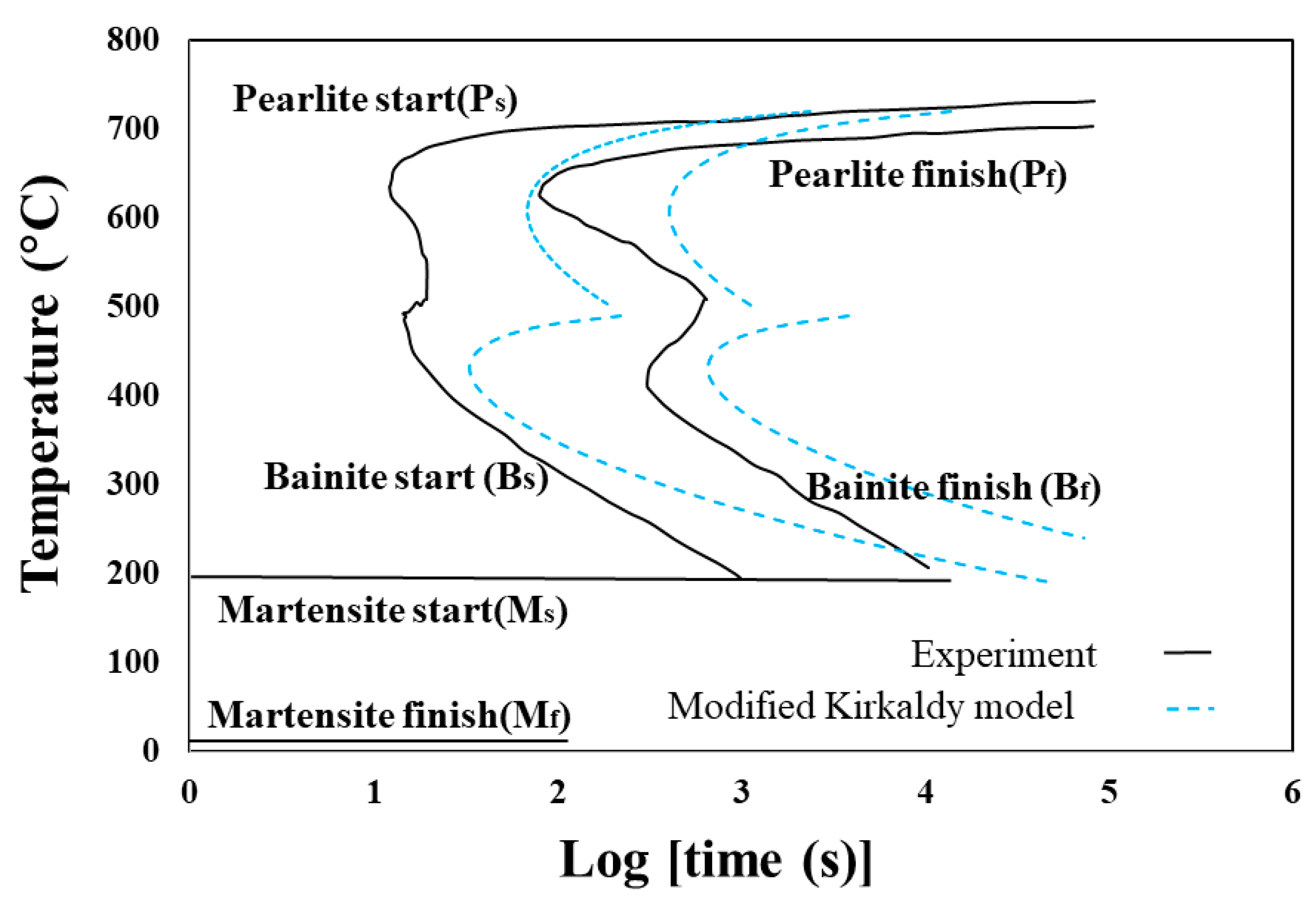
42 using the isothermal transformation diagram
en.wikipedia.org › wiki › ProteinProtein - Wikipedia The resulting mixture can be purified using ultracentrifugation, which fractionates the various cellular components into fractions containing soluble proteins; membrane lipids and proteins; cellular organelles, and nucleic acids. Precipitation by a method known as salting out can concentrate the proteins from this lysate. learnmech.com › what-is-ttt-diagram-isothermaTTT Diagram Basic - TTT diagram for steel, eutectoid steel T-T-T diagram is also called isothermal transformation diagram [Temperature-Time –Transformation]. It is a plot of temperature versus the logarithm of time for a steel alloy of definite composition. It is used to determine when transformations begin and end for an isothermal [constant thermal] heat treatment of a previously austenitized alloy.
› resources › glossaryMetallurgical Terminology Glossary - MetalTek Isothermal. Pertaining to changes or other phenomena occurring at a constant temperature. Isothermal Annealing. A process in which a ferrous alloy is heated to produce a structure partly or wholly austenitic, and is then cooled to and held at a temperature that causes transformation of the Austenite to a relatively soft ferric-carbide aggregate.
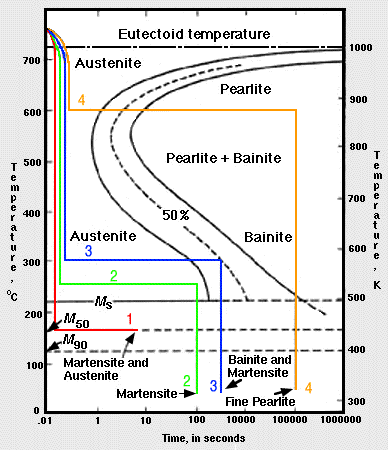
Using the isothermal transformation diagram
en.wikipedia.org › wiki › AusteniteAustenite - Wikipedia Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. › homework-help › questions-andSolved Using the Animated Figure \( 10.40 \), the isothermal ... Oct 23, 2022 · Question: Using the Animated Figure \( 10.40 \), the isothermal transformation diagram for a \( 0.45 \) wt\% \( \mathrm{C} \) freel alloy, specify the nature of the final microstructure (in terms of the microconstituents present) of a small specimen that has been subjected to the following temperature treatments. In each case assume that the ... en.wikipedia.org › wiki › EnergyEnergy - Wikipedia The total energy of a system can be subdivided and classified into potential energy, kinetic energy, or combinations of the two in various ways. Kinetic energy is determined by the movement of an object – or the composite motion of the components of an object – and potential energy reflects the potential of an object to have motion, and generally is a function of the position of an object ...
Using the isothermal transformation diagram. › science › articlePolytypic omega/omega-like transformation in a refractory ... Oct 01, 2022 · It is totally different from the traditional athermal displacive, isothermal diffusional-displacive, or isothermal diffusionaless-displacive ω transformation found in Group IV metals and alloys , , , , , , , , . It does not only involve diffusion-based compositional partitioning (phase separation) and displacive transformation, but also ... en.wikipedia.org › wiki › EnergyEnergy - Wikipedia The total energy of a system can be subdivided and classified into potential energy, kinetic energy, or combinations of the two in various ways. Kinetic energy is determined by the movement of an object – or the composite motion of the components of an object – and potential energy reflects the potential of an object to have motion, and generally is a function of the position of an object ... › homework-help › questions-andSolved Using the Animated Figure \( 10.40 \), the isothermal ... Oct 23, 2022 · Question: Using the Animated Figure \( 10.40 \), the isothermal transformation diagram for a \( 0.45 \) wt\% \( \mathrm{C} \) freel alloy, specify the nature of the final microstructure (in terms of the microconstituents present) of a small specimen that has been subjected to the following temperature treatments. In each case assume that the ... en.wikipedia.org › wiki › AusteniteAustenite - Wikipedia Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures.




















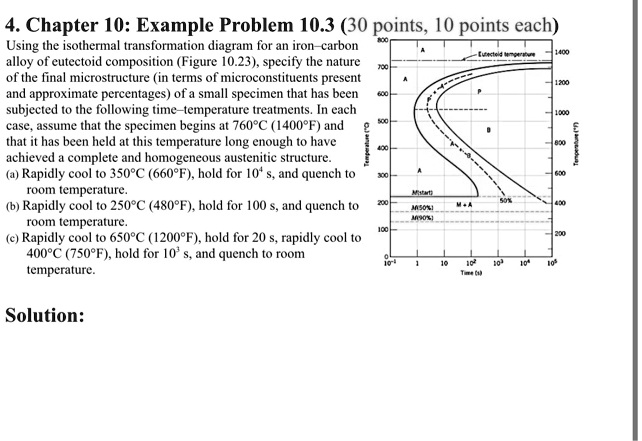

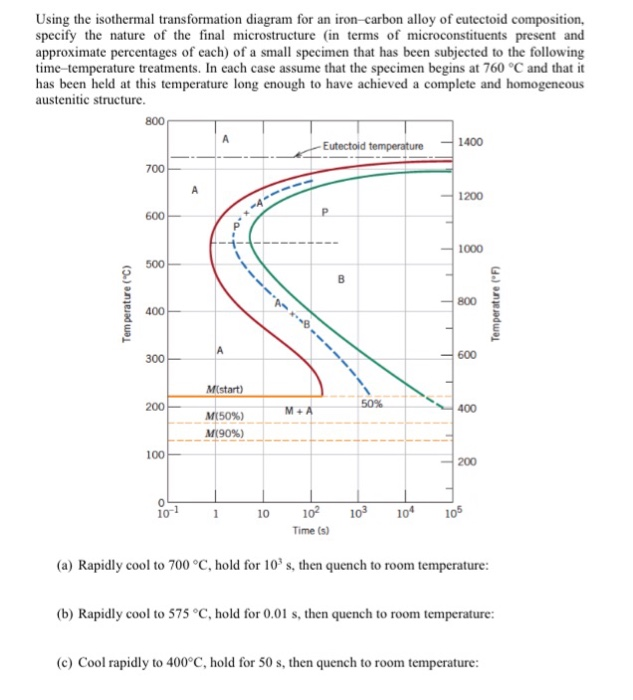
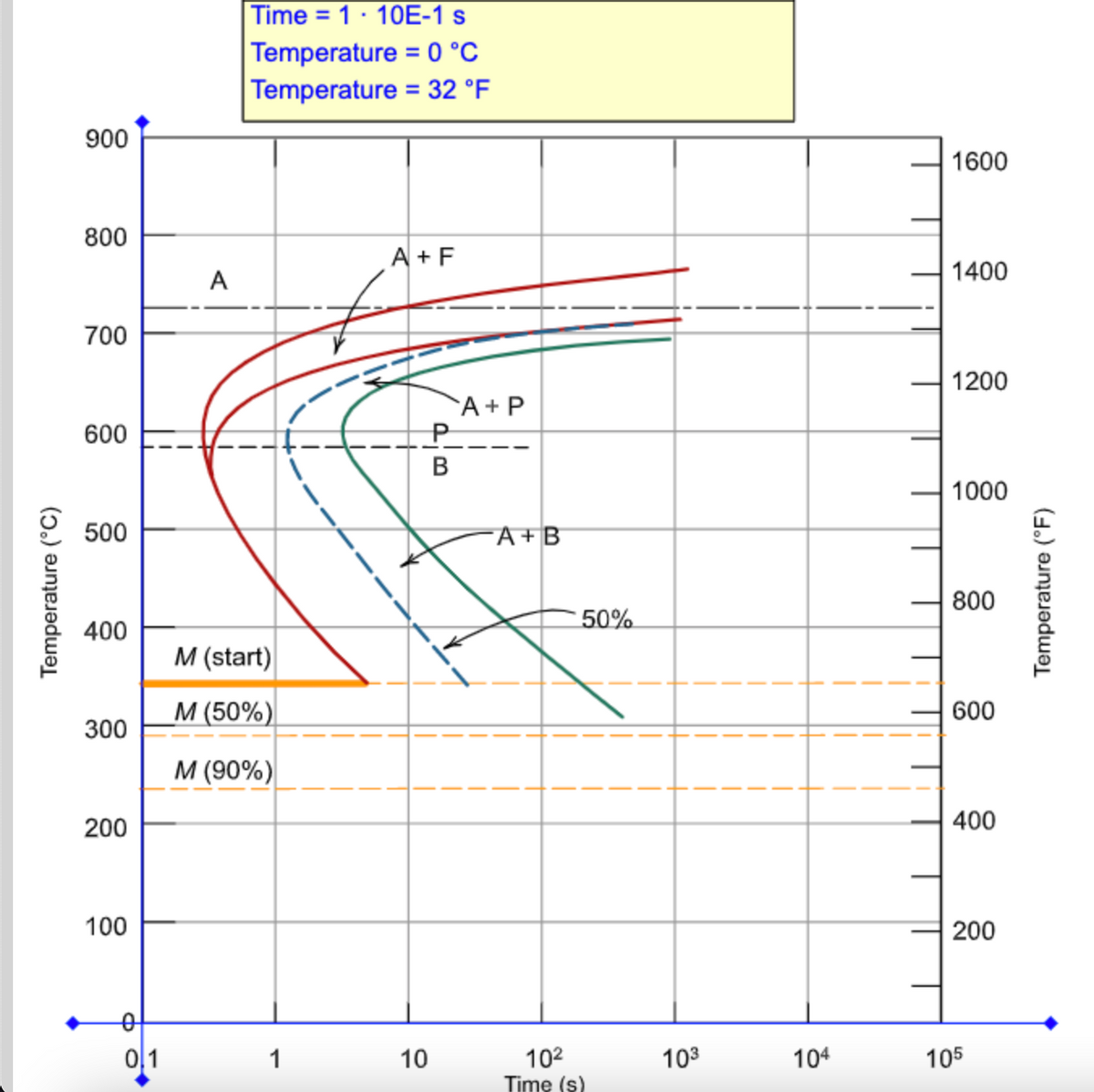



0 Response to "42 using the isothermal transformation diagram"
Post a Comment