41 lithium molecular orbital diagram
Non-flammable ultralow concentration mixed ether electrolyte for ... The lithium dendrite and safety hazard caused by inapposite electrolyte and unstable solid electrolyte interface (SEI) severely hindered practical application of lithium metal batteries (LMBs). ... Based on the frontier molecular orbital theory, ... The diagram of HOMO and LUMO energy levels of DME, HFE and typical Li + coordination structures ... Red giant - Wikipedia Red giants are evolved from main-sequence stars with masses in the range from about 0.3 M ☉ to around 8 M ☉. When a star initially forms from a collapsing molecular cloud in the interstellar medium, it contains primarily hydrogen and helium, with trace amounts of "metals" (in stellar structure, this simply refers to any element that is not hydrogen or helium i.e. atomic number …
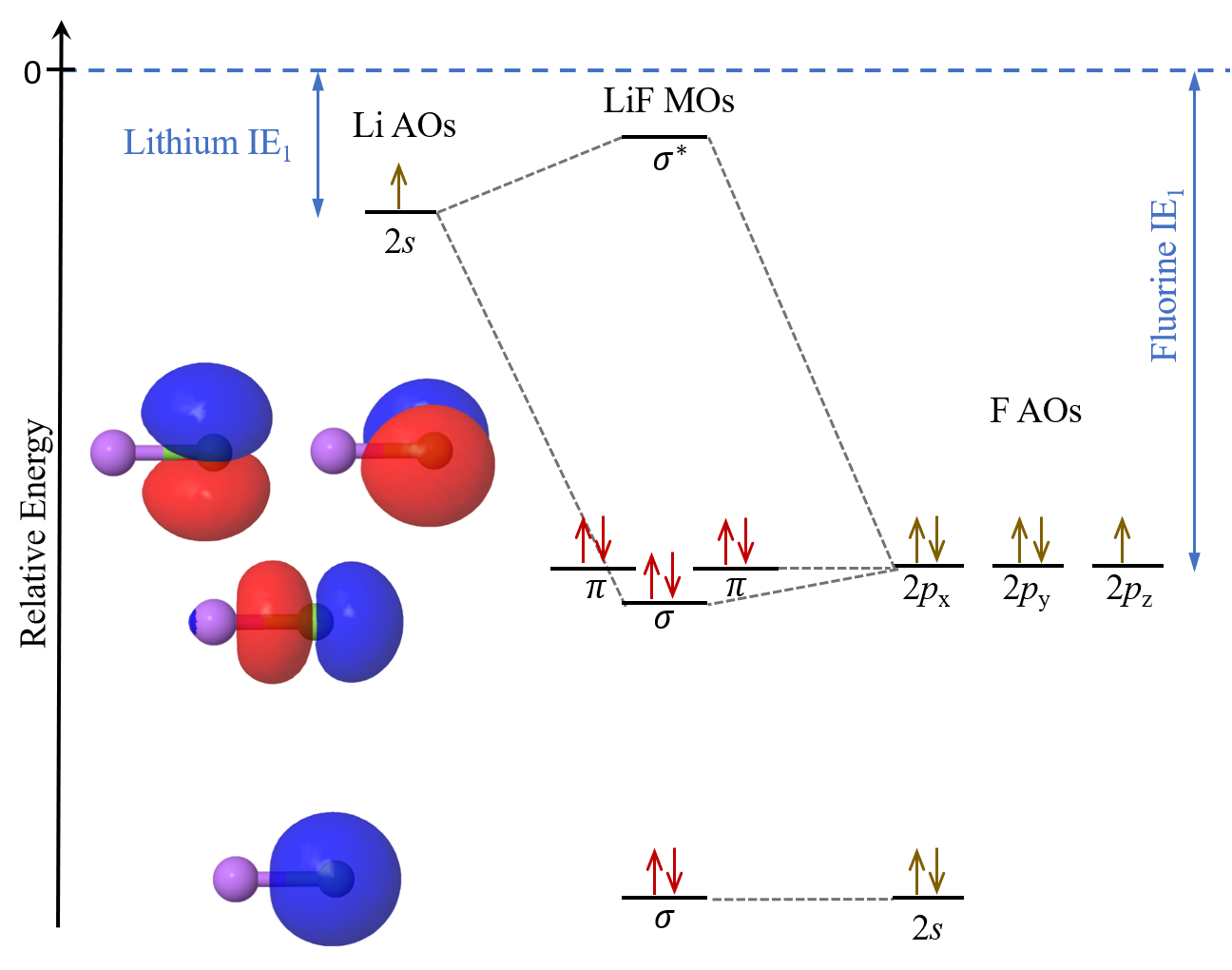
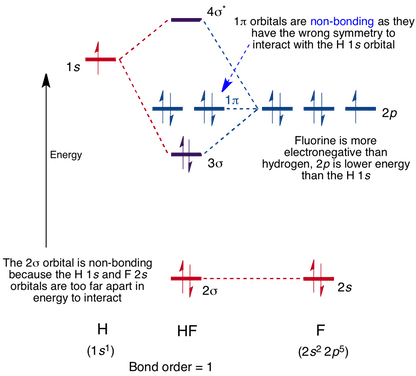
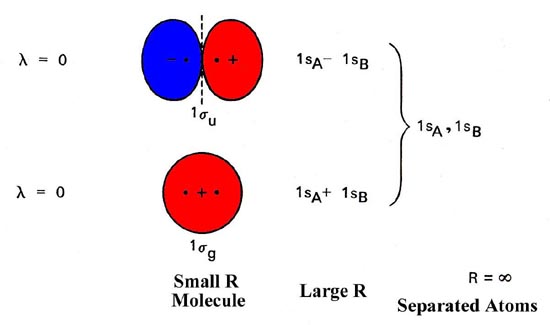
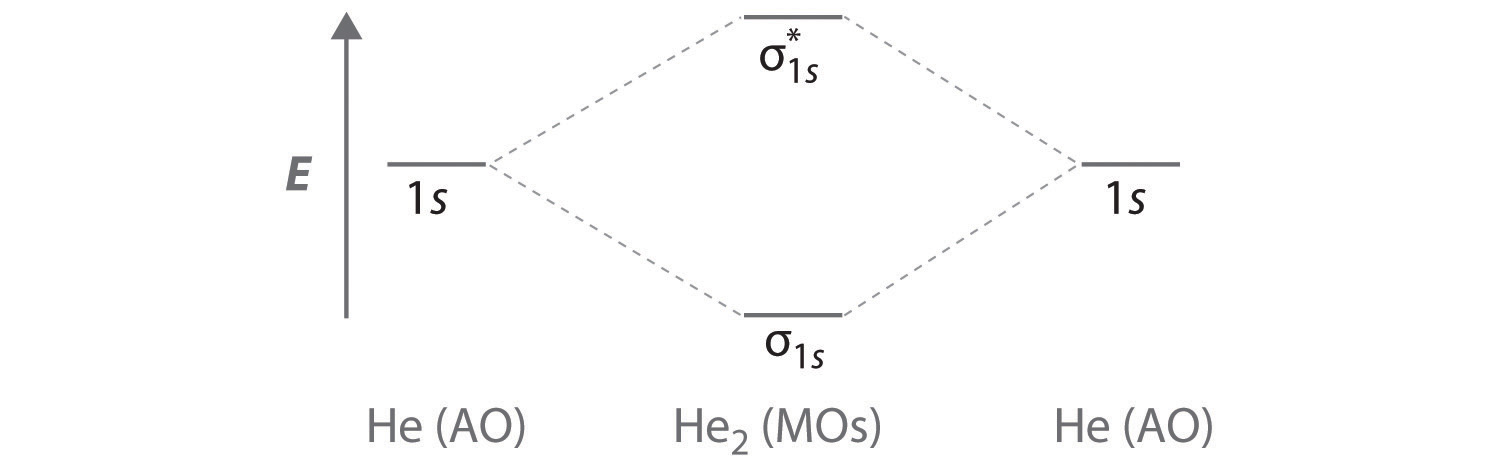
The molecular orbitals of lithium hydride - ECHEMI The occupied atomic orbitals of lithium are 1s and 2s, with energies of around -66 eV and − 5.3 eV respectively. The occupied atomic orbital of hydrogen is 1s, with an energy of -13.6 eV. I believe that it's safe to assume that the 1s orbital of lithium is far too low in energy to interact with the 1s orbital from hydrogen, meaning that only ...

Lithium molecular orbital diagram
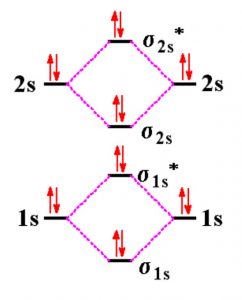
Ionic liquids for high performance lithium metal batteries Aug 01, 2021 · 3. The influence of ionic liquids on electrolyte performance and its application in high energy density batteries. The carbonate-based liquid electrolytes in lithium metal batteries show bad thermal and electrochemical stabilities .Ionic liquid-based electrolytes are promising candidates for lithium metal batteries with high energy density and long-term stability due to … Molecular Orbitals - Chem1 The lithium 1 s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals. Molecular orbital diagram of lithium molecule Li2 - YouTube In this vedio I explained molecular orbital diagram of lithium molecule from the topic molecular orbital theory chapter chemical bonding intermediate first y...
Lithium molecular orbital diagram. 9.8: Molecular Orbital Theory - Chemistry LibreTexts Feb 20, 2022 · Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ... A reflection on lithium-ion battery cathode chemistry - Nature Mar 25, 2020 · b Molecular orbital energy diagram illustrating the lowering of the Fe 2+/3+ redox energy in Fe 2 (SO 4) 3 compared to that in the isostructural Fe 2 (MoO 4) 3, due to a weakening of the Fe–O ... chem.libretexts.org › Bookshelves › General_Chemistry8.4: Molecular Orbital Theory - Chemistry LibreTexts Sep 12, 2022 · Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. This switch in orbital ordering occurs because of a phenomenon called s-p mixing . s-p mixing does not create new orbitals; it merely influences the energies of the existing molecular orbitals. › doi › 10Direct and continuous strain control of catalysts with ... Nov 25, 2016 · The authors acknowledge the help of aberration-corrected STEM-HAADF from P. Yang and Y. Yu at the National Center for Electron Microscopy at the Molecular Foundry. Work at the Molecular Foundry was supported by the DOE Office of Science, Office of Basic Energy Sciences, under contract DE-AC02-05CH11231.
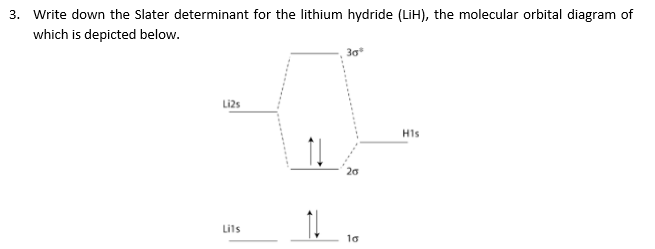
CN- lewis structure, molecular orbital diagram, and, bond order Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3. In the third step, fill the molecular orbitals using the energy and bonding properties of the overlapping atomic orbitals. 4. Draw the MO diagram of CN and fill it with electrons. After that, with the help ... 6.4 Electronic Structure of Atoms (Electron Configurations) The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 6.26 or Figure 6.27). Thus, the electron ... › createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; 2.5.5: Molecular Orbital Diagrams - Chemistry LibreTexts The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. One thing that makes this diagram look different from the ones we have seen previously is that the parent atomic orbitals have widely differing energies; the greater nuclear charge of lithium reduces the energy of its 1 s ...
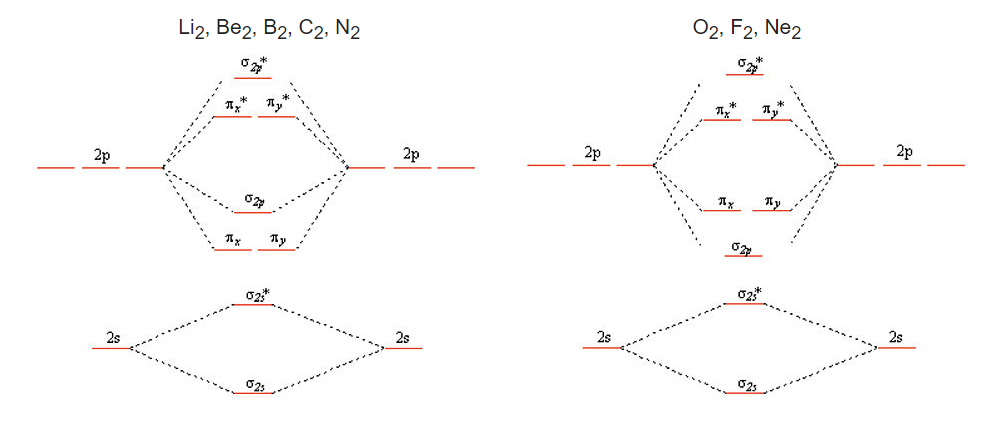
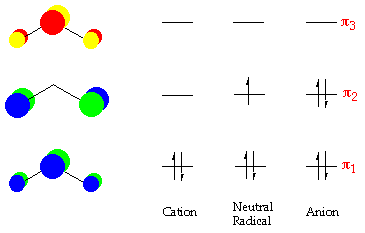
MOT | Molecular Orbital Energy level Diagram for Li2, Li2+ , Li2 ... This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o... How to Calculate Valency | Sciencing Feb 10, 2020 · Valency is a measure of the ability of an atom to bond with other atoms. The higher the number of valent electrons, the more reactive the atom or molecule is. Electrons will occupy the most stable position first. The inner orbital holds up to 2 electrons. The next orbital holds up to 8 electrons. 7.7 Molecular Orbital Theory - Chemistry Fundamentals molecular orbital diagram ( Figure 7.7.9 ). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center. Molecular orbital energy diagrams for homonuclear diatomic species (up ... Conclusion. Molecular orbital energy diagrams help us understand the electronic configuration of elements using quantum mechanics.Molecular orbital is appropriate for simple diatomic molecules like hydrogen,lithium etc,but difficult for polyatomic molecules like ethane,methane etc.A horizontal baseline is present at the centre.Atomic orbitals above baseline have high energy whereas atomic ...
Solved For the diagram below, label each molecular orbital - Chegg For the diagram below, label each molecular orbital (MO). lithium MO's lithium 2p - - 2p E 2s - 2s [Review Topics] [References] Use the References to access important values if needed for this que Fill in the Molecular Orbital Energy Diagram for the diatomic molecule Hz. hydrogen MO's hydrogen 2s 2s E 2s c*ls 1s 1s G1s Fill in the Molecular ...
topblogtenz.com › cyanide-cn-lewis-structureCN- lewis structure, molecular orbital diagram, and, bond order Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3. In the third step, fill the molecular orbitals using the energy and bonding properties of the overlapping atomic orbitals. 4. Draw the MO diagram of CN and fill it with electrons. After that, with the help ...

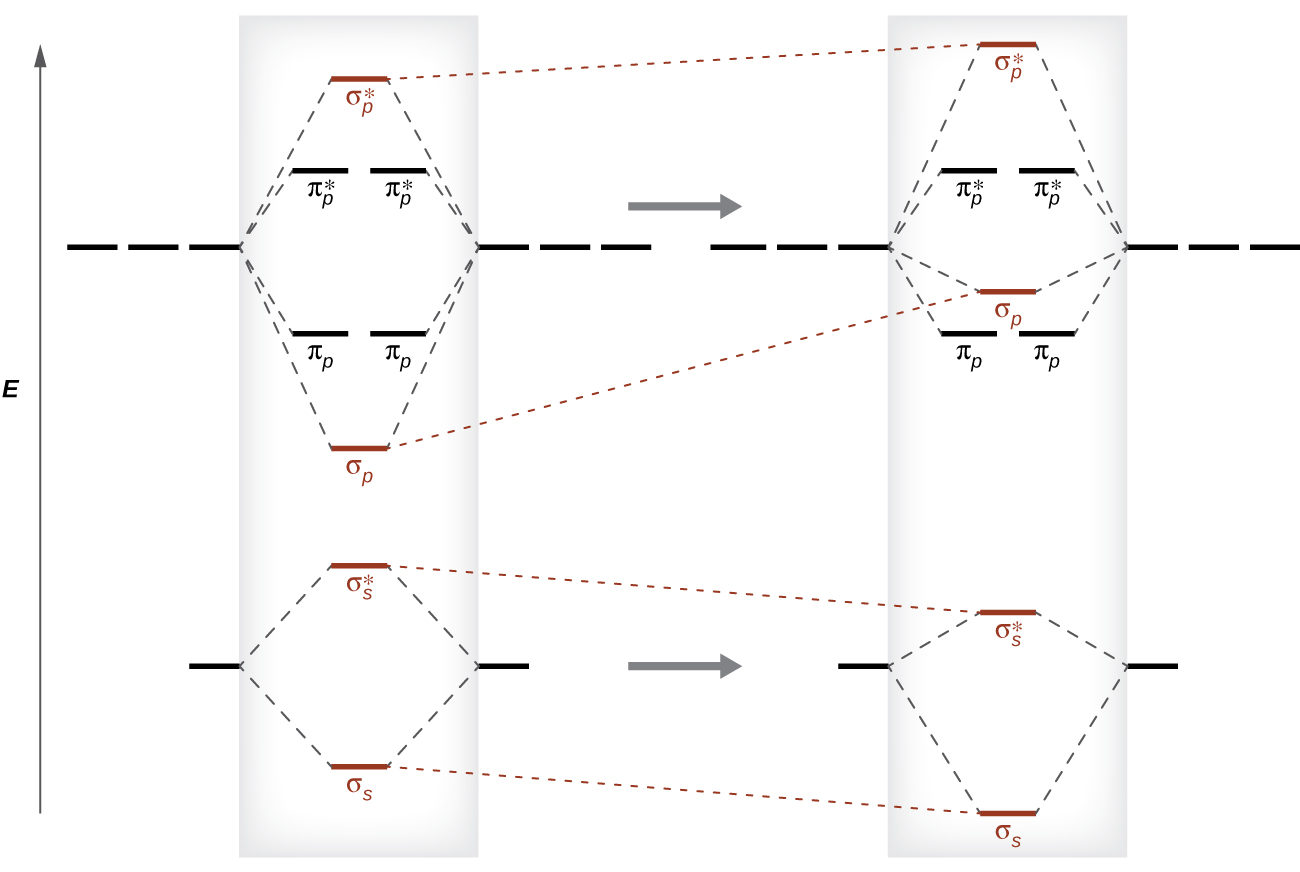
the molecular orbital diagrams for two and four atom linear chains of lithium atoms are shown in f 2
The molecular orbitals of lithium hydride - Chemistry Stack Exchange I also understand that the atomic orbitals have to be quite close in energy for any significant interaction to take place. The occupied atomic orbitals of lithium are 1s and 2s, with energies of around -66 eV and − 5.3 e V respectively. The occupied atomic orbital of hydrogen is 1s, with an energy of -13.6 eV.
chem.libretexts.org › Bookshelves › General_Chemistry9.8: Molecular Orbital Theory - Chemistry LibreTexts Feb 20, 2022 · Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ...
8 - Drawing Molecular Orbital Diagrams — Flux Science Nov 12, 2021 · Molecular Orbital Diagram. As we’ve established, bonding and antibonding interactions are the key to molecular orbital theory. In fact, the idea of molecular orbitals, the distribution of electrons across a molecule, arises from how electrons distribute themselves energetically. ... In the second period, only lithium to nitrogen undergo sp ...
en.wikipedia.org › wiki › Milankovitch_cyclesMilankovitch cycles - Wikipedia The semi-major axis of the orbital ellipse, however, remains unchanged; according to perturbation theory, which computes the evolution of the orbit, the semi-major axis is invariant. The orbital period (the length of a sidereal year) is also invariant, because according to Kepler's third law, it is determined by the semi-major axis. Longer-term ...
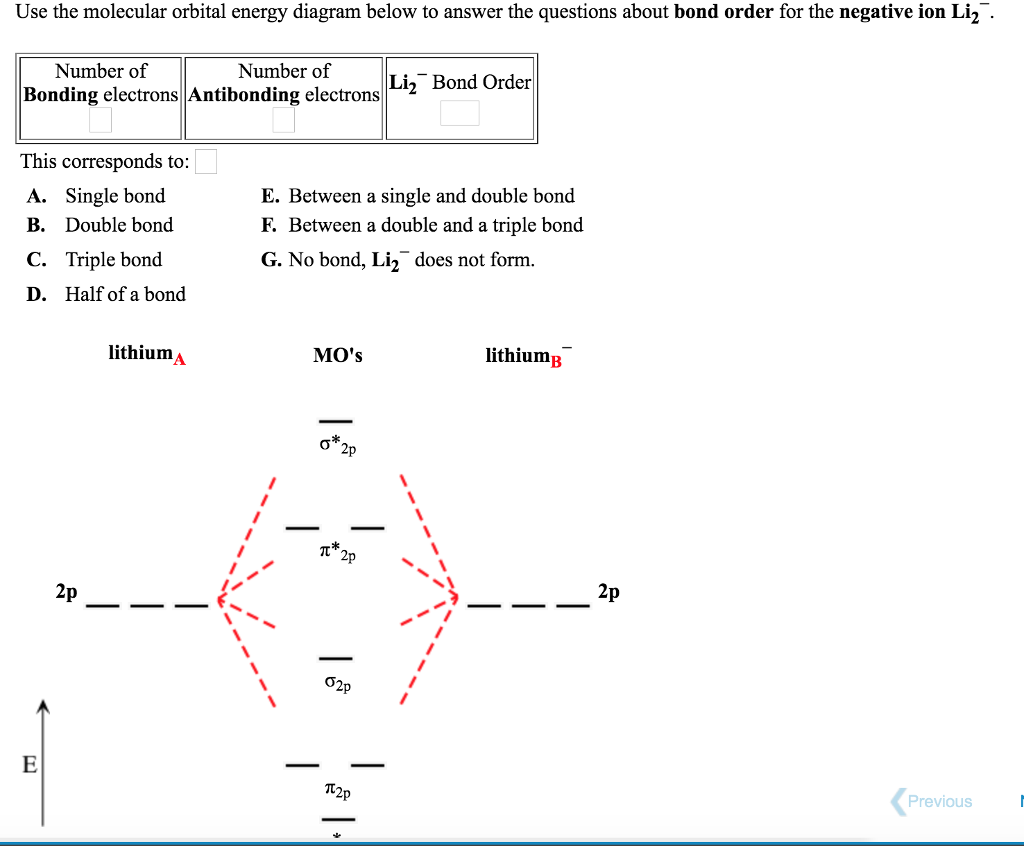
OneClass: The molecular-orbital diagrams for two- and four-atom linear ... The molecular-orbital diagrams for two- and four-atom linear chains of lithium atoms are shown in Figure 12.22. Construct a molecular-orbital diagram for a chain containing six lithium atoms and use it to answer the following questions: (a) How many molecular orbitals are there in the diagram?
Semiconductors - Types, Examples, Properties, Application, Uses It is the highest occupied molecular orbital at absolute zero. The charge carriers in this state have their own quantum states and generally do not interact with each other. ... The energy band diagram of an intrinsic semiconductor is shown below: (a) Intrinsic Semiconductor at T = 0 Kelvin, behaves like an insulator (b) At t>0, four thermally ...
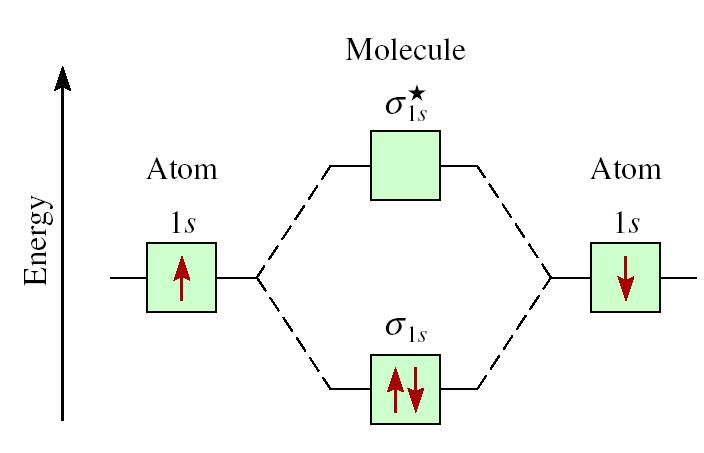
Lithium molecular orbitals - Big Chemical Encyclopedia The band of molecular orbitals formedby the 2s orbitals of the lithium atoms, described above, is half filledby the available electrons. Metallic beryllium, with twice the number of electrons, might be expected to have a full 2s band . If that were so the material would not exist, since the anti-bondinghalf of the band would be fully occupied.
[Solved] The molecular orbitals of lithium hydride | 9to5Science Formation of the molecular orbitals also changes the energy level of the core orbitals even though these do not participate appreciably in the bonding. This is because what I call "reverse shielding" occurs. In a lithium atom in the ground state, there are of course two 1s electrons and one 2s electron.
Lithium(Li) electron configuration and orbital diagram - Valenceelectrons Lithium orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Solved The molecular-orbital diagrams for two- and four-atom - Chegg The molecular-orbital diagrams for two- and four-atom linear chains of lithium atoms are shown in Figure 12.23 in the textbook. Construct a molecular-orbital diagram for a chain containing eight lithium atoms and use it to answer the following questions. How many molecular orbitals are there in the diagram?
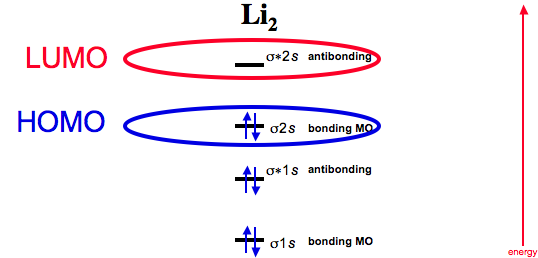
Molecular Orbital Diagram of Lithium Molecule - YouTube Molecular Orbital Diagram of Lithium Molecule - Nature of Chemical Bond - Chemistry Class 11 4,589 views Sep 27, 2018 88 Dislike Ekeeda 855K subscribers Molecular Orbital Diagram of Lithium...
Li2- Molecular Orbital Diagram Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital.
Molecular Orbital Diagram of li2 | Bond order of lithium | Is Li2 ... 1,507 views Premiered Jun 10, 2020 Molecular Orbital energy level Diagrams of Li2 according to the molecular orbital theory explains the bond order and magnetic properties of lithium mol ...more...
How to Write the Orbital Diagram for Lithium (Li) - YouTube To write the orbital diagram for the Lithium atom (Li) first we need to write the electron configuration for just Li. To do that we need to find the number ...
What is the orbital diagram of lithium? - Answers Orbital diagram for lithium? the atomic formula of lithium is 3 . so the electronic concentration will be 2,1 . this means that there will be 2 electrons in first shell n 1 electron in second shell...
› flux › mods8 - Drawing Molecular Orbital Diagrams — Flux Science The way these bonds are placed on any molecular orbital diagram is according to how the atomic orbitals that make the MOs mix. In that mixing, there are two factors to consider: (1) atomic symmetry and (2) mixing. Image via Chegg Symmetry As a bond occurs, a bond or internuclear axis - the line that connects the nuclei of two bonded atoms - forms.
Molecular orbital diagram of lithium molecule Li2 - YouTube In this vedio I explained molecular orbital diagram of lithium molecule from the topic molecular orbital theory chapter chemical bonding intermediate first y...
Molecular Orbitals - Chem1 The lithium 1 s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
Ionic liquids for high performance lithium metal batteries Aug 01, 2021 · 3. The influence of ionic liquids on electrolyte performance and its application in high energy density batteries. The carbonate-based liquid electrolytes in lithium metal batteries show bad thermal and electrochemical stabilities .Ionic liquid-based electrolytes are promising candidates for lithium metal batteries with high energy density and long-term stability due to …




















0 Response to "41 lithium molecular orbital diagram"
Post a Comment