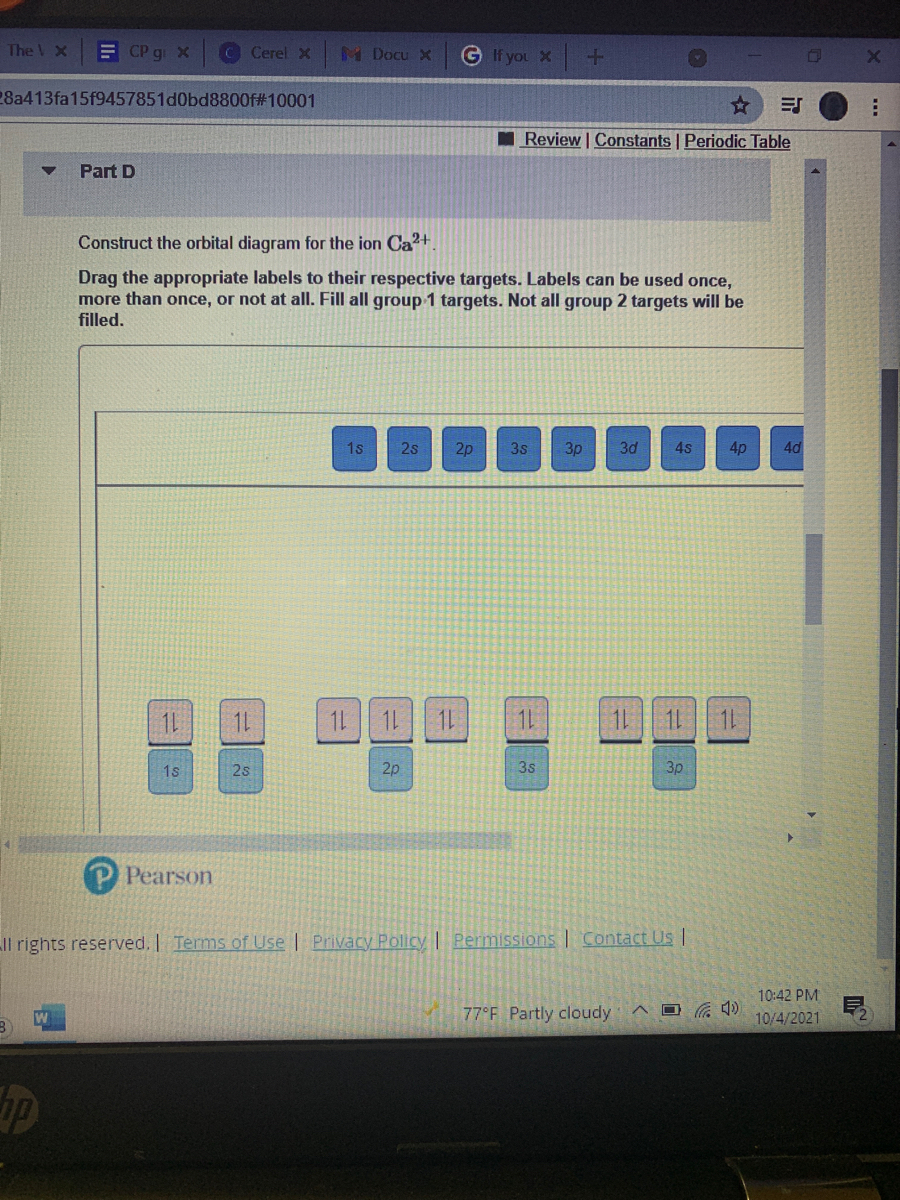
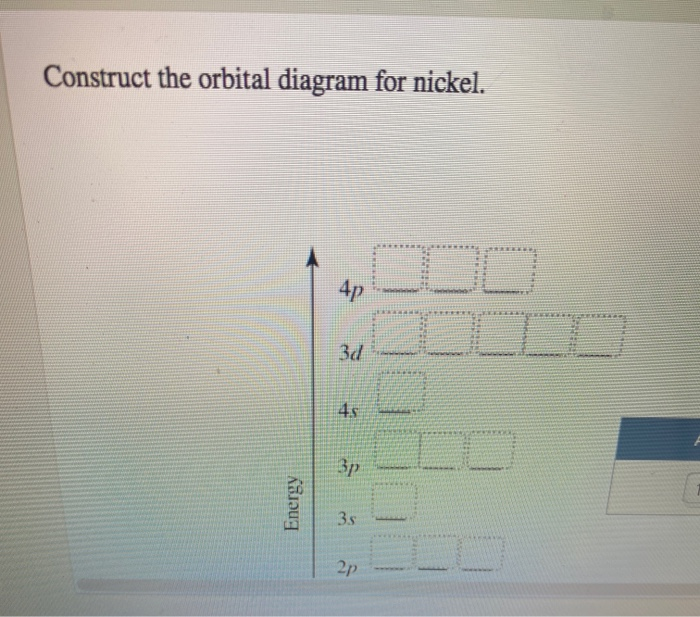
41 construct the orbital diagram for ni.
How do you write the electron configuration for #Ni^(+2)#? - Socratic.org Explanation: Electron configuration for Ni is 1s22s22p64s23d8. N i2+ has two electrons less than Ni ( that is why N i2+ is positively charged). So when writing electron configuration for N i2+ we exclude last two electrones from the last shell of Ni electron confgiruation. I suggest to watch Tyler DeWitt's YouTube video on electron configuration. Construct the orbital diagram for nickel. | Chegg.com Science. Chemistry. Chemistry questions and answers. Construct the orbital diagram for nickel.
Answered: Draw the molecular orbital diagram for… | bartleby Solution for Draw the molecular orbital diagram for [Ti(H2O)6]³+ with the electrons filled in the orbitals. Clearly label the bonding and anti-bonding orbitals. ... The atomic number of Ni is 28 and its valence shell electronic configuration is 3d84s2.Ni is in +2 ...

Construct the orbital diagram for ni.
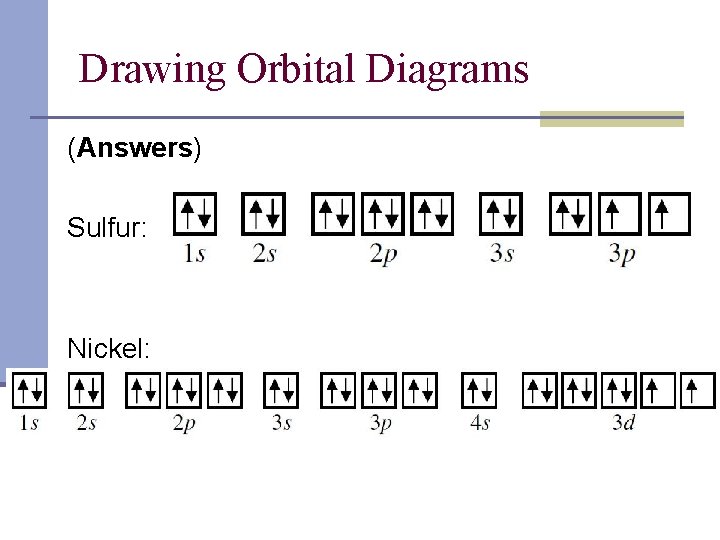
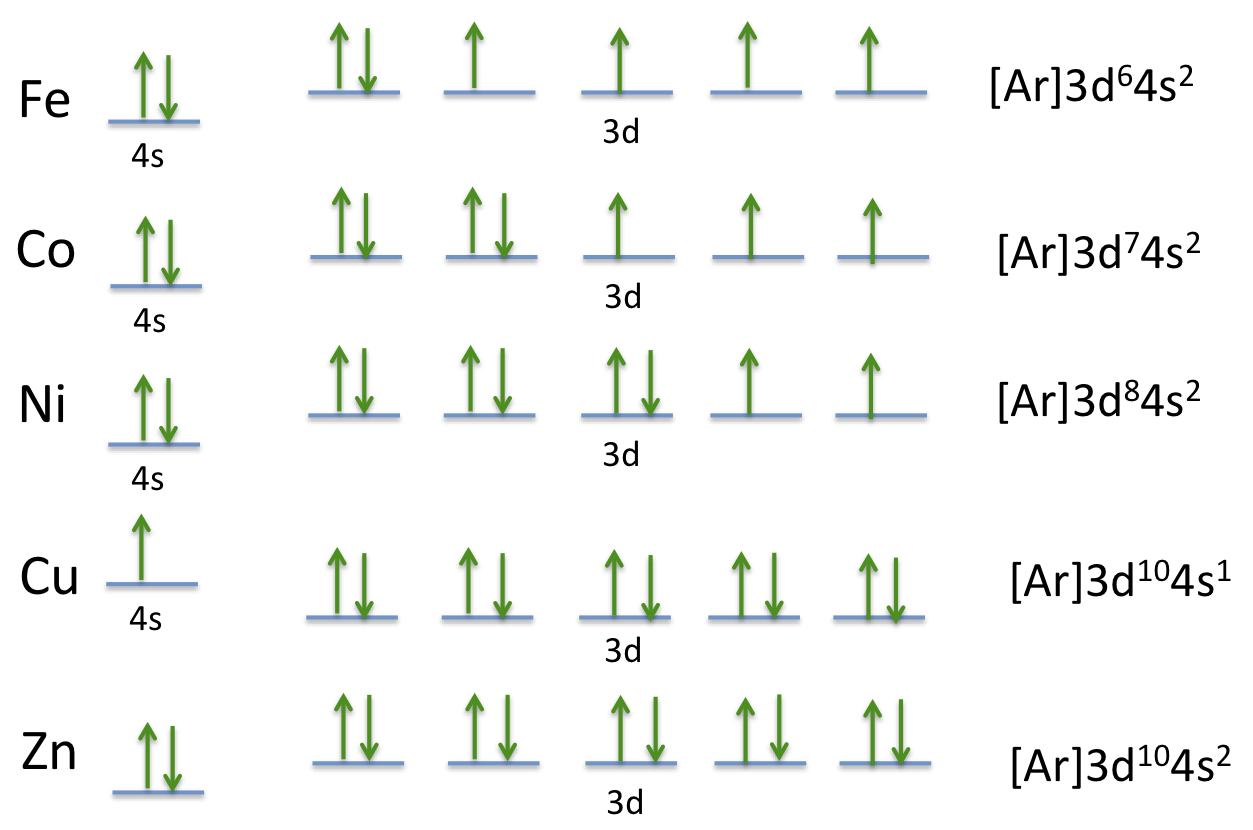
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital ... How to Write the Atomic Orbital Diagram for Nickel (Ni) 1,801 views Dec 21, 2021 To write the orbital diagram for the Nickel (Ni) first we need to write the electron configuration for just Ni. To do that we need to find the number of electrons for the... What is the orbital diagram for helium? - NSN search Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining 2 electrons for Be go in the 2s orbital. Therefore the Be electron configuration will be 1s22s2.
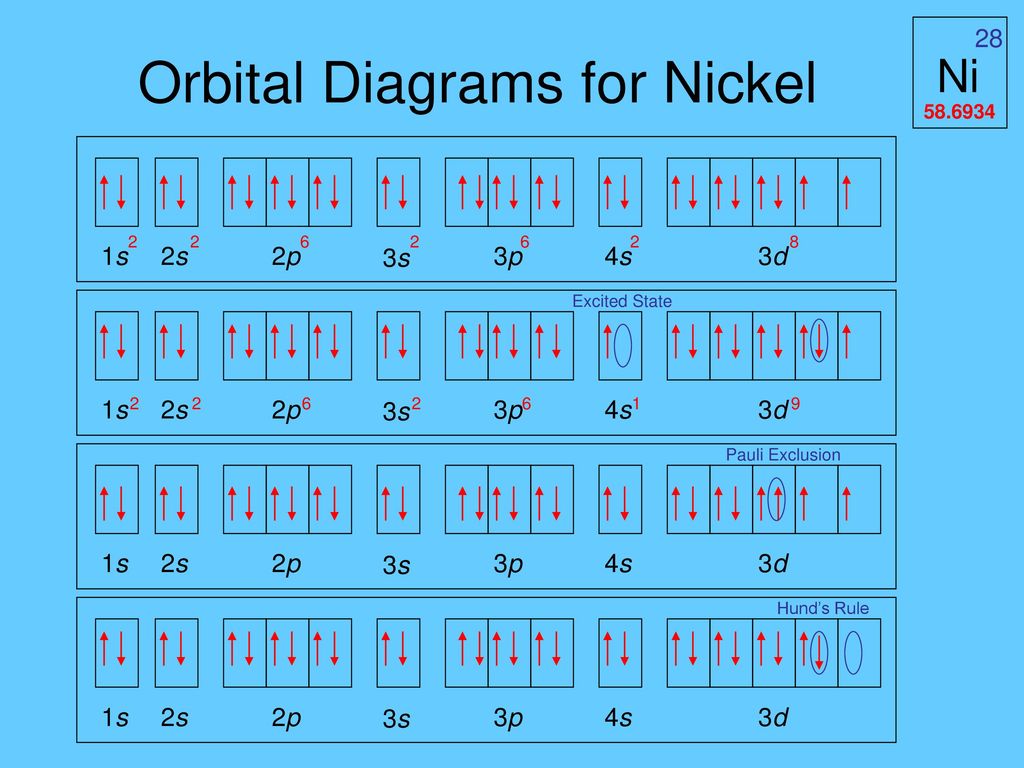
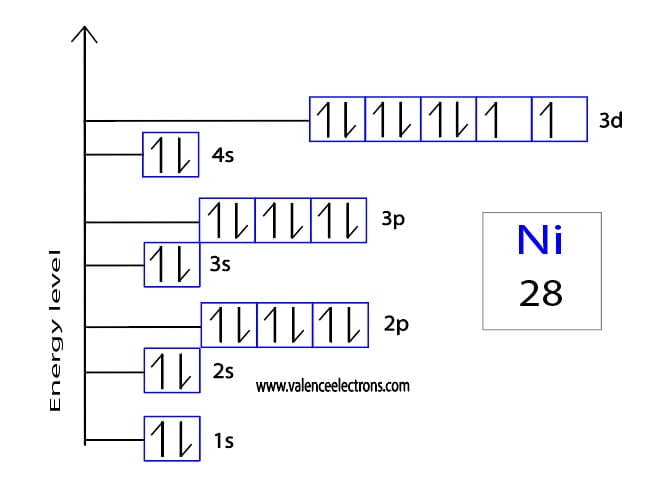
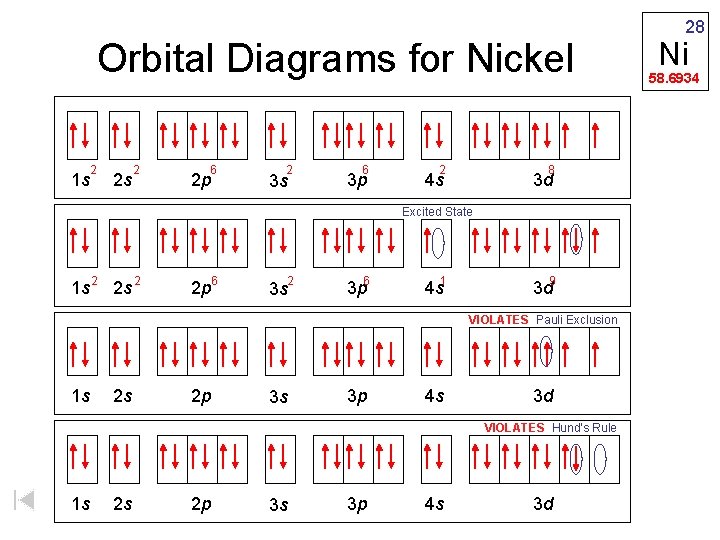
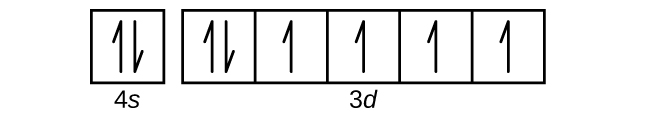
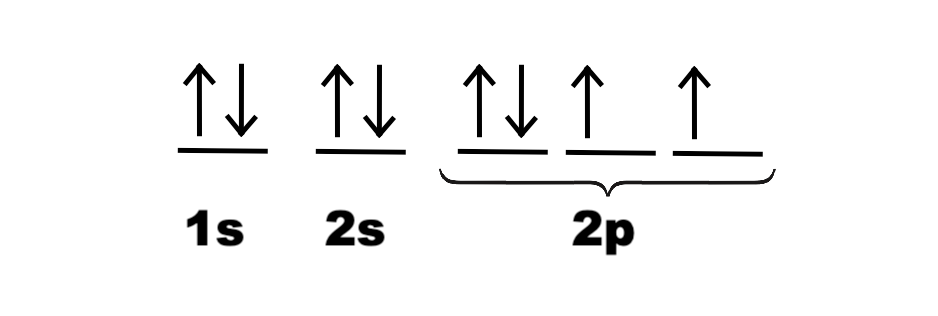
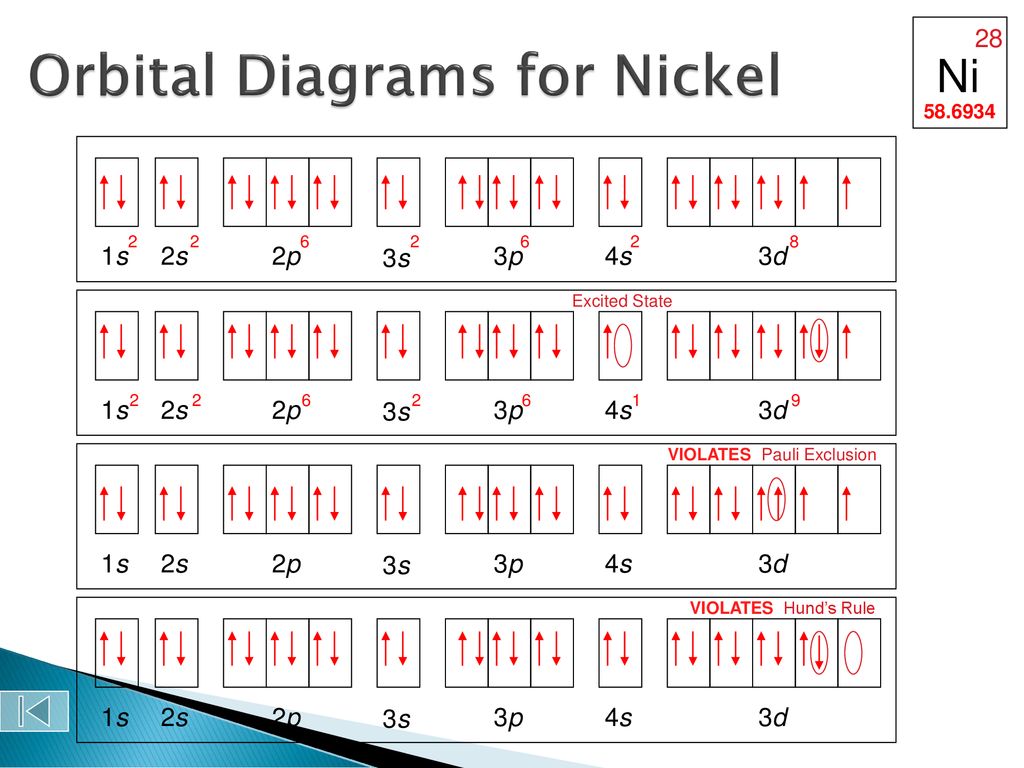
Construct the orbital diagram for ni.. Draw the orbital diagram for nickel (Ni). How many unpaired - Kunduz Draw the orbital diagram for nickel (Ni). How many unpaired electrons does Ni have and is Ni paramagnetic or diamagnetic? 0; paramagnetic 0; diamagnetic 2; paramagnetic 2; diamagnetic 3; paramagnetic. Show Answer. Create an account. Get free access to expert answers. Construct The Orbital Diagram For Ni Answer to Construct the orbital diagram for Ni. Start by adding the appropriate subshells. For example, carbon is in the 2p block.1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. Nickel(Ni) electron configuration and orbital diagram - Valenceelectrons To write the orbital diagram of nickel (Ni), you have to do the electron configuration of nickel. Which has been discussed in detail above. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. Nickel orbital diagram What is the orbital diagram for nickel? - Answers What is the correct orbital diagram would be for the element nickel? Before you can make a diagram, you will need to know the atomic number. With the atomic number you can make the diagram, which...
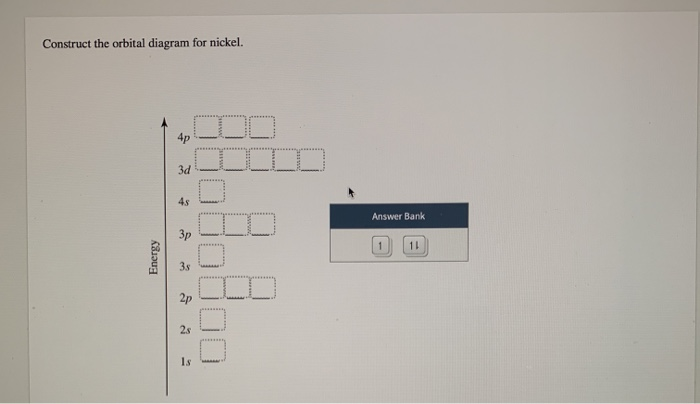
Electron Configurations and Orbital Diagrams Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like C (carbon), Mg (magnesium), S (sulfur) and more. What is the orbital diagram for nickel? - Quora Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev... Nickel Orbital Diagram - Learnool Step #3: draw orbital diagram of nickel Let's break down each step in detail. #1 Find Electrons of Nickel The atomic number of nickel represents the total number of electrons of nickel. Since the atomic number of nickel is 28, the total electrons of nickel are 28. #2 Write Electron Configuration of Nickel Solved Construct the orbital diagram for nickel. Answer Bank - Chegg Expert Answer. Transcribed image text: Construct the orbital diagram for nickel. Answer Bank 111 Energy.
Answered: Draw an orbital diagram, write a full… | bartleby Solution for Draw an orbital diagram, write a full electron configuration and core configuration for nickel. Construct the orbital diagram for Ni. - ZuoTi.Pro The atomic number of nickel is 28. So, there are 28 electrons in nickel atom. First start filling the electrons with lowest energy orbital and each orbital is singly occupied with one electron. And only two electrons with opposite spin can fit into an orbital. Thus, orbital diagram of nickel is as follow. Ans: Use Frost's circle to construct orbital energy diagrams for | Quizlet The languages of valence-bond theory and molecular orbital theory are commonly combined when discussing unsaturated organic compounds. Construct the molecular orbital energy level diagrams of ethene on the basis that the molecule is formed from the appropriately hybridized C H 2 \mathrm{CH}_{2} CH 2 or CH fragments. Draw complete orbital diagrams for (a) As and (b) Ni. - YouTube Draw complete orbital diagrams for (a) As and (b) Ni.Watch the full video at: ...
Draw the orbital diagram for Ni and label each of the orbitals ... The number of protons can be found in the periodic table as the atomic number. To get the number of neutrons, subtract the protons from the atomic mass. Next, you draw the orbitals, and their...
Copper Orbital Diagram - Learnool Here's how you can draw the orbital diagram of copper step by step. Step #1: find electrons of copper. Step #2: write electron configuration of copper. Step #3: draw orbital diagram of copper. Let's break down each step in detail.
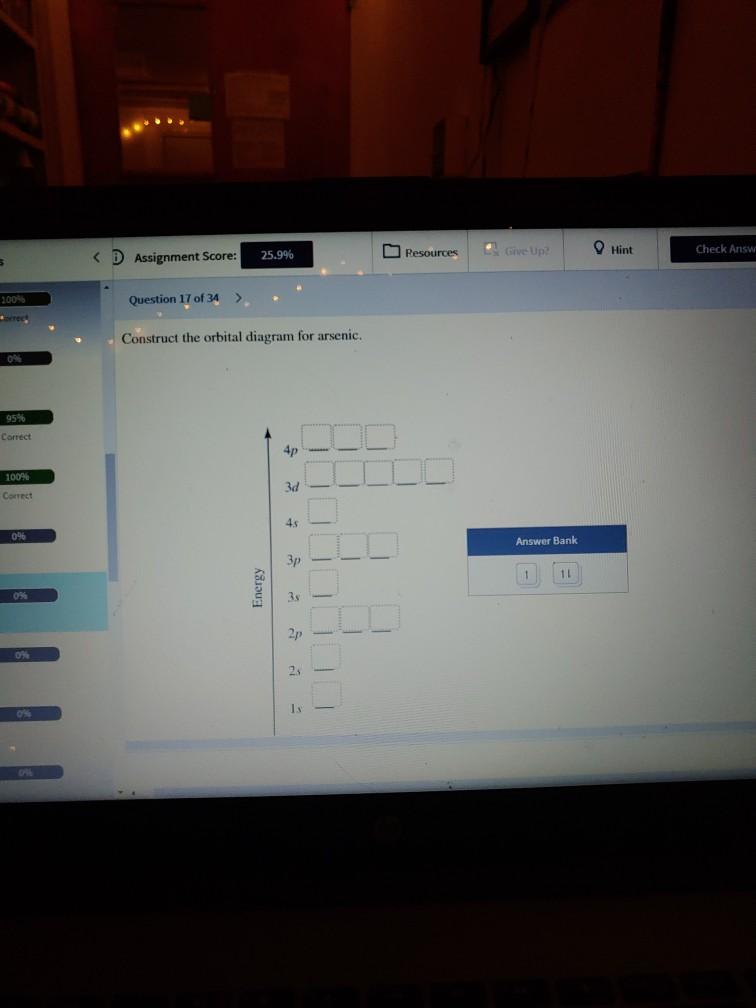
(Get Answer) - Construct the orbital diagram for arsenic. 3d 1000 ... Question: Construct The Orbital Diagram For Nickel. WOOD QUID Answer Bank 3 OTO Energy Answer Bank Energy 180 A E 11 Question: Construct The Orbital Diagram For Nickel. WOOD QUID Answer Bank 3 OTO Energy Answer Bank Energy 180 A E 11 Posted 5 months ago. Q: Below Is The Orbital Diagram For Arsenic (33As), Which Has A Ground Electron ...
Draw orbital diagrams for the shorthand configurations of - Numerade Draw a little diagram for abbreviated configuration of so first one it's nickel so are gone. Yeah. Mhm. For a stool? Hello? So 11 11 one. It's three D . Download the App! Get 24/7 study help with the Numerade app for iOS and Android! Enter your email for an invite. ... Draw orbital diagrams for the abbreviated configurations of (a) $\mathrm{Ni
Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s22s22p3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Nitrogen(N) electron configuration and orbital diagram - Valenceelectrons To write the orbital diagram of nitrogen (N), you have to do the electron configuration of nitrogen. Which has been discussed in detail above. Nitrogen orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital.
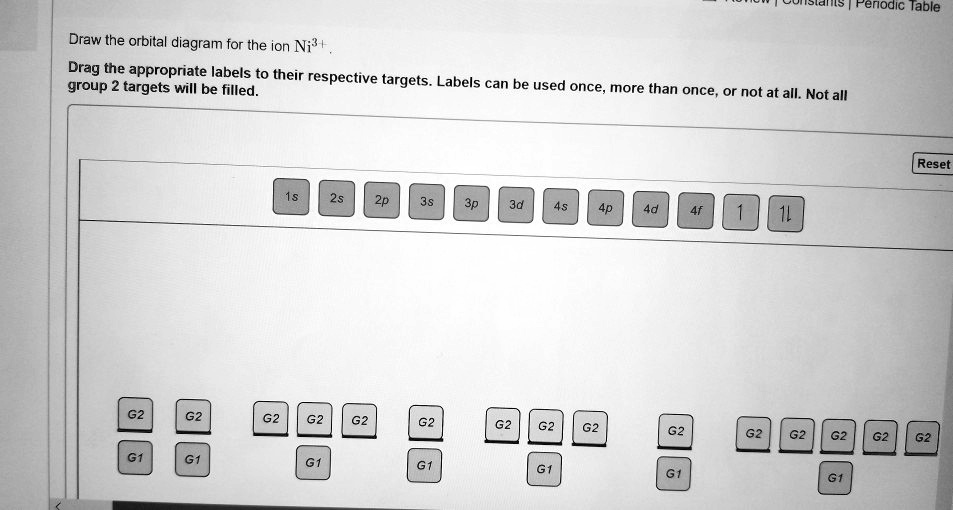
Cr3+ Orbital Diagram In writing orbital diagrams, first, determine the electron configuration of the neutral atom and remove. ... Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. Solution for Draw an orbital diagram with boxes as shown in Figure 1 for the Zn2+, Cu2 ...
Nitrogen Orbital diagram, Electron configuration, and ... - Topblogtenz The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows-
What is the orbital diagram for helium? - NSN search Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining 2 electrons for Be go in the 2s orbital. Therefore the Be electron configuration will be 1s22s2.
How to Write the Atomic Orbital Diagram for Nickel (Ni) 1,801 views Dec 21, 2021 To write the orbital diagram for the Nickel (Ni) first we need to write the electron configuration for just Ni. To do that we need to find the number of electrons for the...
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital ...















![ANSWERED] Draw the orbital diagram for nickel (Ni ...](https://media.kunduz.com/media/sug-question/raw/53143403-1659267658.4356651.jpeg)





0 Response to "41 construct the orbital diagram for ni."
Post a Comment