40 nitrogen molecular orbital diagram
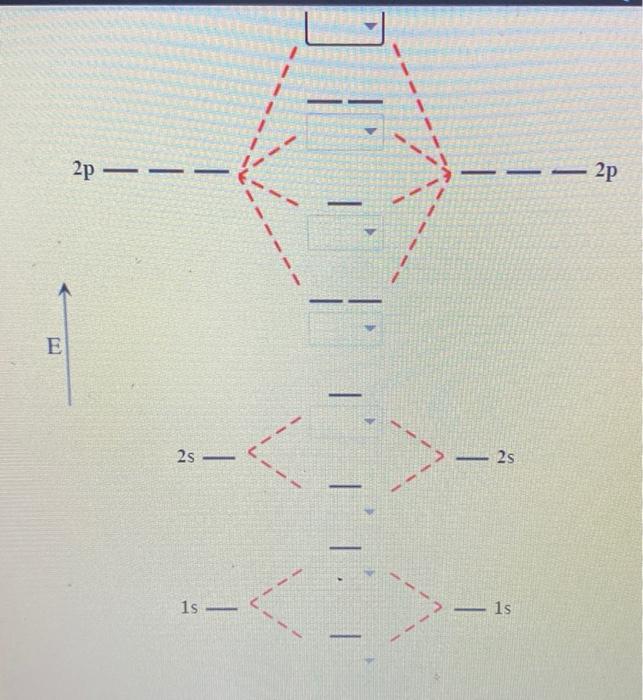
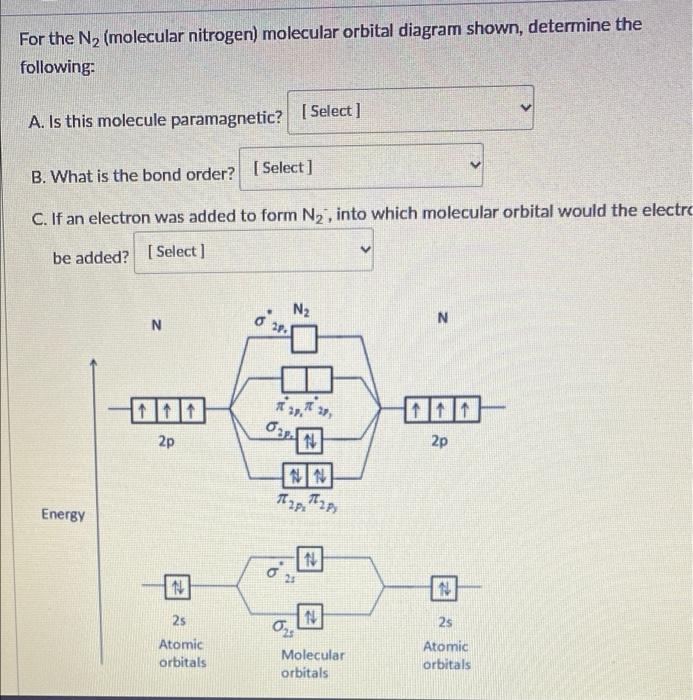
8 - Drawing Molecular Orbital Diagrams — Flux Science Molecular orbital diagram of diatomic nitrogen. Homonuclear molecular orbitals are formed between two elements that are the same, meaning that they are naturally symmetrical and will perfectly overlap. However, before we fill out this diagram, compare this MOD to the one above, particularly in the 2p region. Solved For the N2 (molecular nitrogen) molecular orbital | Chegg.com Question: For the N2 (molecular nitrogen) molecular orbital diagram shown, determine the following: A. Is this molecule paramagnetic? (Select] B. What is the bond order? [Select] C. If an electron was added to form N2, into which molecular orbital would the electro be added? [ Select ] N2 N N 27. 2p IN 2p NN h2 h2p Energy N 2s N. 25 Atomic ...
CN- lewis structure, molecular orbital diagram, and, bond order Nitrogen needs 6 more electrons to complete its octet rule as it already shares 2 electrons with a single bond. So, after putting 6 valence electrons on nitrogen, we are left with 2 electrons more. ... Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 ...

Nitrogen molecular orbital diagram
Molecular orbital energy level diagrams -Hydrogen, Hypothetical ... The molecular orbital energy level diagram of He2 (hypothetical) is given in Fig. Here, Nb = 2 and Na = 2 Bond order = Nb - Na / 2 = 2 - 2 / 2 = 0. As the bond order for He2 comes out to be zero, this molecule does not exist. 3. Nitrogen molecule (N2). The electronic configuration of nitrogen (Z=7) in the ground state is 1s2 2s2 2p1x 2p1y 2p1z . Molecule - Wikipedia Molecular science. The science of molecules is called molecular chemistry or molecular physics, depending on whether the focus is on chemistry or physics.Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties. Orbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.
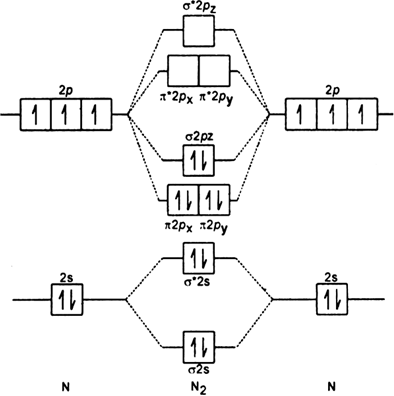
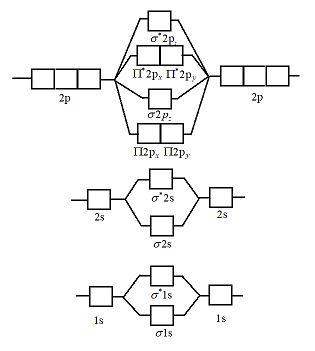
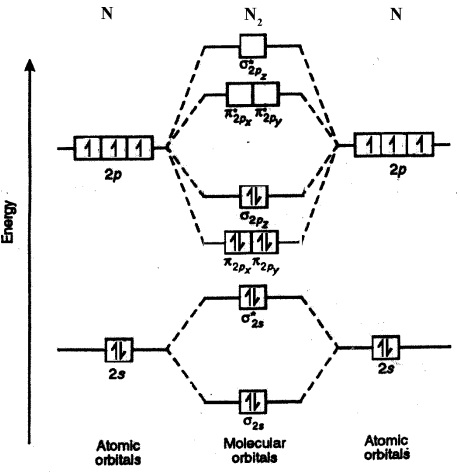
Nitrogen molecular orbital diagram. Lewis structure - Wikipedia Nitrogen is the least electronegative atom of the two, so it is the central atom by multiple criteria. Count valence electrons. Nitrogen has 5 valence electrons; each oxygen has 6, for a total of (6 × 2) + 5 = 17. The ion has a charge of −1, which indicates an extra electron, so the total number of electrons is 18. Connect the atoms by ... Explain the formation of nitrogen molecule by molecular orbital theory ... Non bonding - Electrons in non-bonding orbitals are associated with atomic orbitals that do not interact with one another, hence they do not participate in bond formation. Formation of Nitrogen molecule by Molecular Orbital Theory: Electronic configuration of Nitrogen (Z = 7) = 1 s 2 2 s 2 2 px 1 2 py 1 2 pz 1. When two Nitrogen atom combine ... Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). Energy level diagram for Molecular orbitals - Class Notes The double bond in C2 consist of both Pi bonds because the four electrons are present in the two pi molecular orbitals. 10) N2 The atomic number of nitrogen is 7 The electronic configuration of N2 is KK (σ (2s))2 (σ∗(2s))2 (π (2px))2 (π (2py))2 (σ (2pz))2 Nb= 8, Na= 2 Bond Order= 3 Bond order value of 3 means that N2 contains a triple bond.
Nitrogen(N) electron configuration and orbital diagram - Valenceelectrons Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. We already know that the p-subshell has three orbitals. Solved Below is a molecular orbital diagram for nitrogen - Chegg Chemistry questions and answers. Below is a molecular orbital diagram for nitrogen monoxide. Dran-and-drop the orbital designations with the targets on the orbital diagram. 2s 2s. Question: Below is a molecular orbital diagram for nitrogen monoxide. Dran-and-drop the orbital designations with the targets on the orbital diagram. 2s 2s. What is the molecular orbital diagram for NO? - Quora Your MO diagram for NO should look like this: O is more electronegative than N so its orbitals are slightly lower in energy and the bonding orbitals are slightly more concentrated on O. Note the odd electron is in a Pi*2p orbital. Now draw two more MO diagrams for NO+ and NO- Nitrogen - Wikipedia Nitrogen gas is an industrial gas produced by the fractional distillation of liquid air, or by mechanical means using gaseous air (pressurised reverse osmosis membrane or pressure swing adsorption). Nitrogen gas generators using membranes or pressure swing adsorption (PSA) are typically more cost and energy efficient than bulk delivered nitrogen.
Why is the molecular orbital diagram for O2 different from N2? Since Oxygen atom has 8 electrons, the molecular orbitals of Oxygen molecule (O 2) has 16 electrons, which are distributed as below : Molecular orbital energy level diagram of O 2 • Bond order = (10 - 6)/2 = 2 (O = O) What is the bond order of N2 and O2? Molecular Nitrogen and Related Diatomic Molecules Here is the full molecular orbital diagram for N2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively. N2O Lewis Structure, Molecular Geometry ... - Techiescientist N2O or nitrous oxide is commonly known as laughing gas. There are several other names by which this compound is known like sweet air, protoxide of nitrogen, etc. N2O is a colorless gas with a molecular weight of 44.013 g/mol. The boiling point of this compound is -88.48℃ and the melting point is -90.86℃. Molecular Orbital Theory: Concept & Diagram | StudySmarter Let's look at the molecular orbital diagram for N 2. Nitrogen has an electron configuration of 1s 2 2s 2 2p 3. Since Nitrogen is a period 2 element, we will start the molecular orbital diagram with 2s. First, fill in the atomic orbital diagram for both nitrogen atoms. Each nitrogen atom will have two electrons in the 2s, and then 1 electron in ...
Molecular Orbital Diagram of Nitrogen Molecule - YouTube Molecular Orbital Diagram of Nitrogen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET...
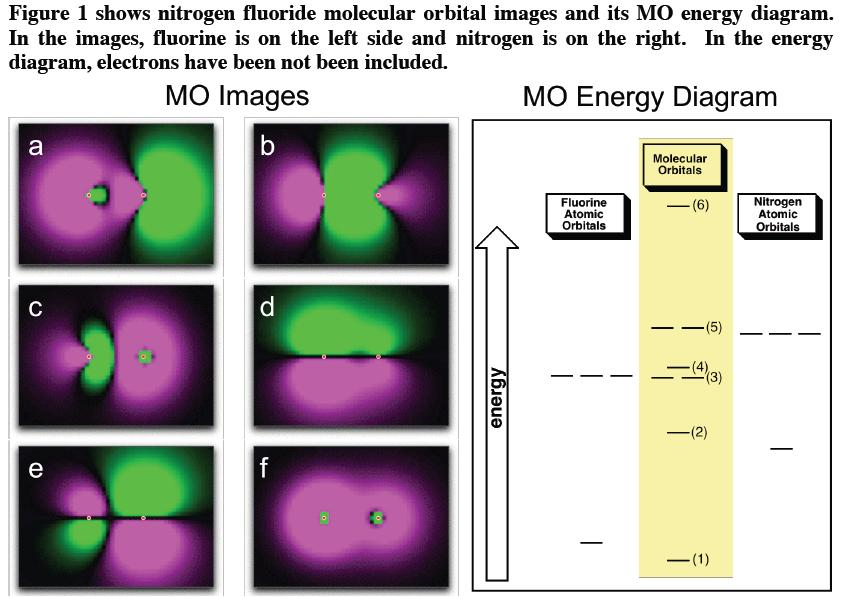
Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation ... The π-orbitals are not the HOMO, instead it is the σ lone pair at nitrogen. The next more complicated is N O X −, which is isoelectronic to O X 2. In this case it is hard to find a less symmetric isoelectronic version. It is well known, that the ground state is a triplet because of the degeneracy of the π-orbitals.
Molecular orbitals in Nitrogen - ChemTube3D There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two perpendicular pi bonds. Three filled bonding orbitals… … and three empty antibonding orbitals.
HCN Lewis Structure, Molecular Geometry, Hybridization, MO ... Oct 19, 2022 · In the case of HCN, let us look at how the atomic orbitals fuse to make molecular orbitals. Electronic configuration of C is 2s2 2p2, electronic configuration of H is 1s1, and electronic configuration of N is 2s2 2p3. Here, one sp orbital of C fuses with 1s orbital of H. And the other sp orbital of C fuses with one of the p orbitals of Nitrogen.
Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
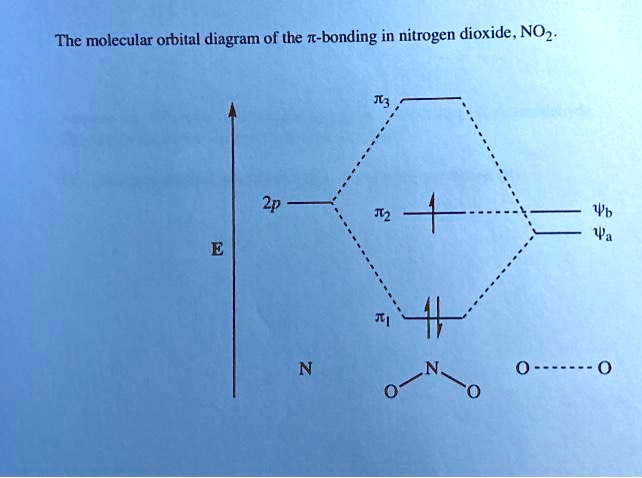
Nitrogen Dioxide - Beloit College Nitrogen Dioxide. Molecular orbitals in NO 2. Will the molecule be linear or bent? Click on a color picture to watch the geometry change from linear to bent. 2𝝅u. 2b 1. 6a 1. 1𝝅g.
Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration Nitrogen is an element that has 7 electrons and when we talk about the valence electrons then, valence electrons are those electrons that are present in the outer shell and are associated with molecules or an atom and which also can participate in the chemical formation.
Nitrogen molecular orbital diagram - Big Chemical Encyclopedia Describe the bonding of the chlorine bridges in this dimer in molecular orbital terms. 8-6 BF can be obtained by reaction of BF3 with boron at 1850° C and low pressure BF is highly reactive but can be preserved at liquid nitrogen temperature (77 K). Prepare a molecular orbital diagram of BF. How would the molecular orbitals of BF differ from CO, with which BF is isoelectronic ...
orbitals - What is the origin of the differences between the MO schemes ... S-p mixing is the primary cause of the difference in the molecular orbitals of nitrogen and oxygen, which is influenced by the initial atomic orbital energies. The lighter second period elements (prior to oxygen) have a relatively small difference in energy between the 2s and 2p orbitals. This allows sufficient s-p mixing to lower the energy of ...
Why is the molecular orbital diagram for O₂ different from N₂? Answer (1 of 2): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ...
Nitric Oxide Molecular Orbital Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining .. Nitric oxide is a heteronuclear molecule that exhibits mixing. THE MO's FORMED BY TWO 1s ORBITALS. E. Energy of a 1s . MODIFIED ENERGY LEVEL DIAGRAM. 2s. s2s*. 2s. s2s. 2p . NITRIC OXIDE (NO). Number of. Something is not correct with your MO diagram.
Nitrogen Orbital diagram, Electron configuration, and ... - Topblogtenz The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows-
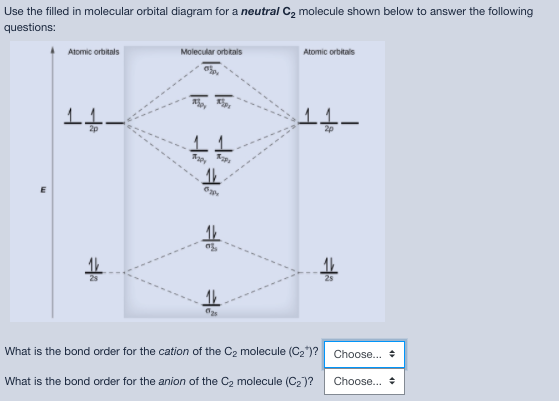
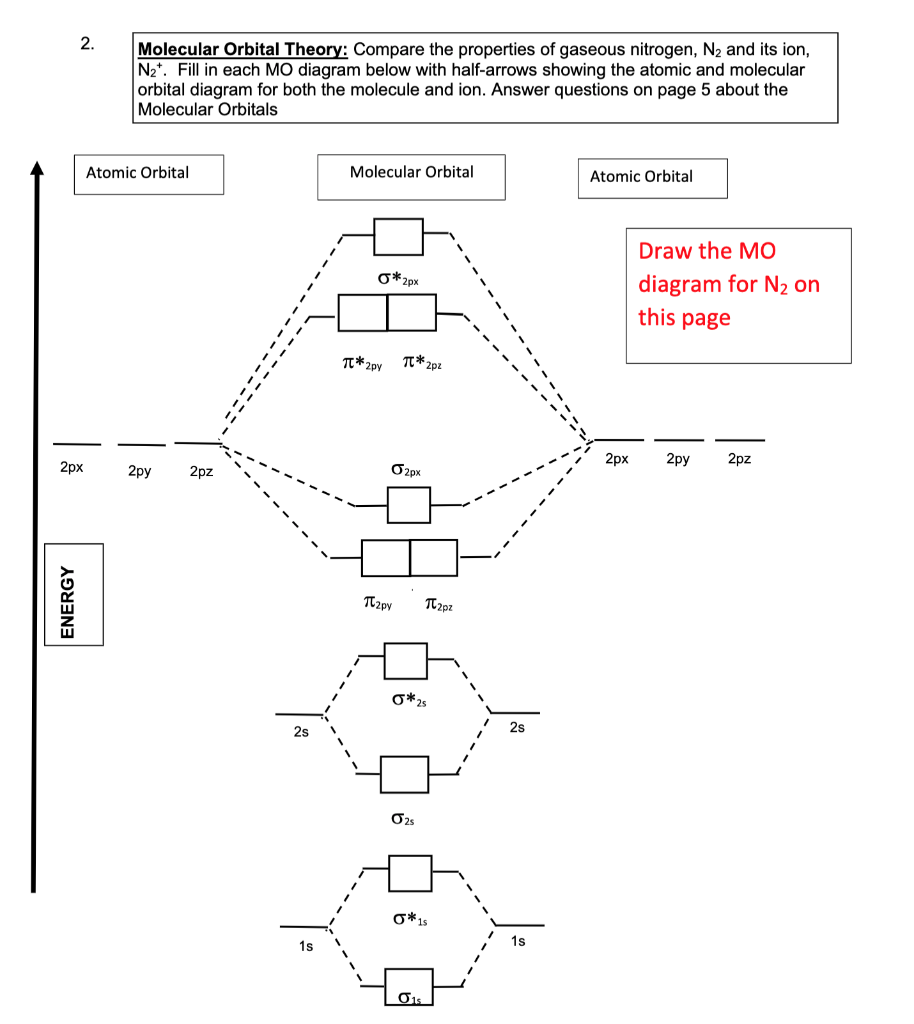
Molecular Orbital Diagrams: Nitrogen, Carbon, and Boron In this video we will draw the molecular orbital diagrams for diatomic nitrogen, carbon and boron. We will also calculate their bond order and determine if they are paramagnetic or diamagnetic....
NO2 Lewis Structure, Molecular Geometry ... - Techiescientist Lewis Structure of NO2. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Oxygen belongs to group 16 ( or group 6) and has an atomic number of 8, therefore a valency of 6.
Orbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.
Molecule - Wikipedia Molecular science. The science of molecules is called molecular chemistry or molecular physics, depending on whether the focus is on chemistry or physics.Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties.
Molecular orbital energy level diagrams -Hydrogen, Hypothetical ... The molecular orbital energy level diagram of He2 (hypothetical) is given in Fig. Here, Nb = 2 and Na = 2 Bond order = Nb - Na / 2 = 2 - 2 / 2 = 0. As the bond order for He2 comes out to be zero, this molecule does not exist. 3. Nitrogen molecule (N2). The electronic configuration of nitrogen (Z=7) in the ground state is 1s2 2s2 2p1x 2p1y 2p1z .





















0 Response to "40 nitrogen molecular orbital diagram"
Post a Comment