39 ethylene molecular orbital diagram
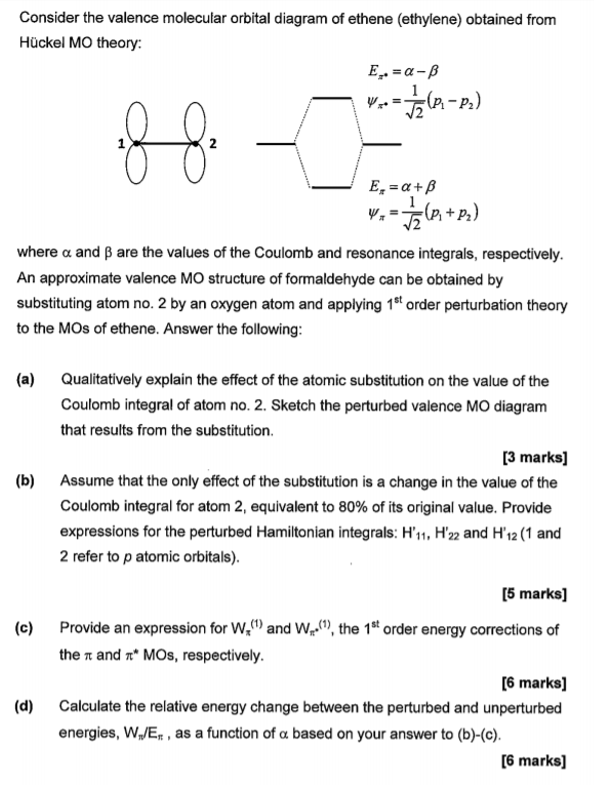
Hybridization of C2H4 - Ethene (Ethylene) - BYJUS In ethylene, each carbon combines with three other atoms rather than four. There is a formation of a sigma bond and a pi bond between two carbon atoms. C2H4 Molecular Geometry And Bond Angles. C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o. Introduction to Pi Molecular Orbitals Ethylene - Chad's Prep® In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals: ψ1 and ψ2*, (also referred to as π1 and π2*).
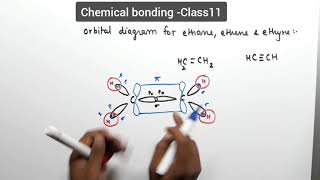
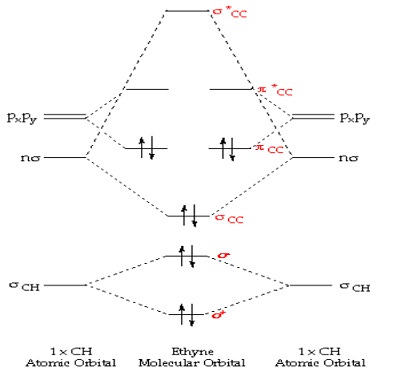
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene) Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene) 42,083 views Mar 12, 2021 976 Dislike Share Save chemistNATE 223K subscribers The carbon atoms of C2H2 are sp hybridized. This makes...

Ethylene molecular orbital diagram
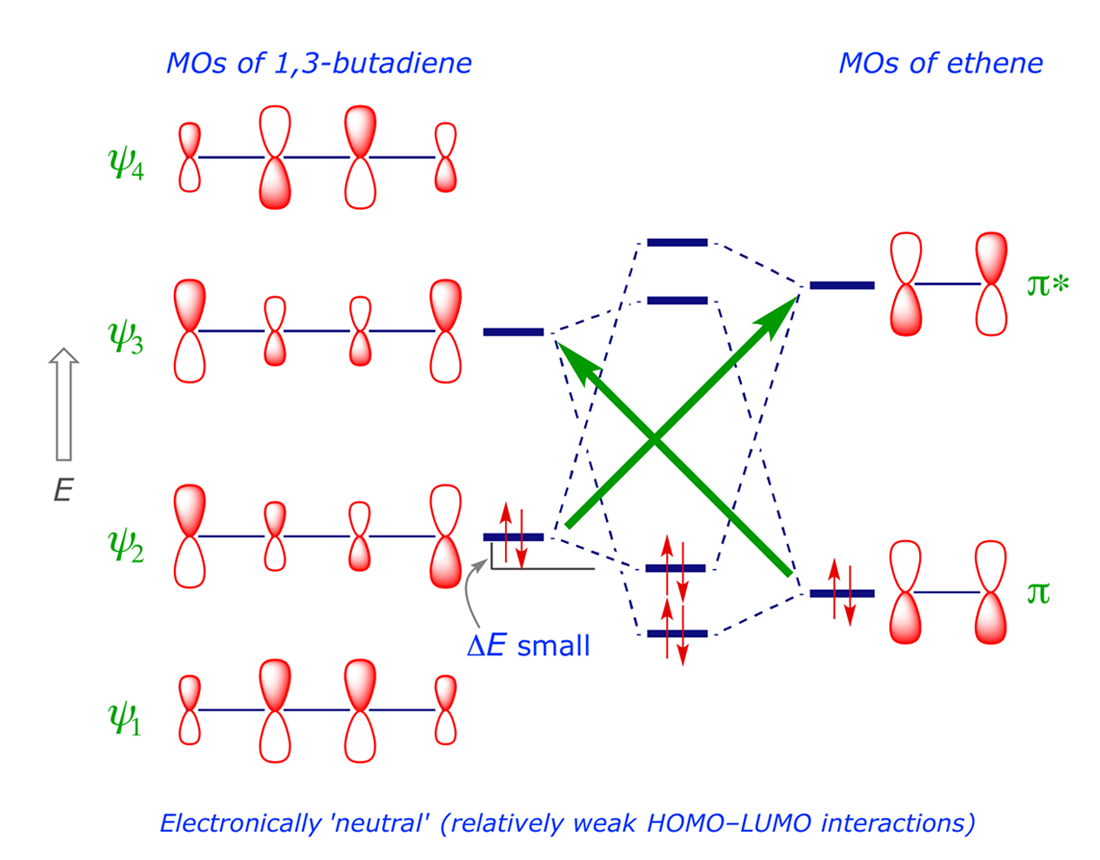
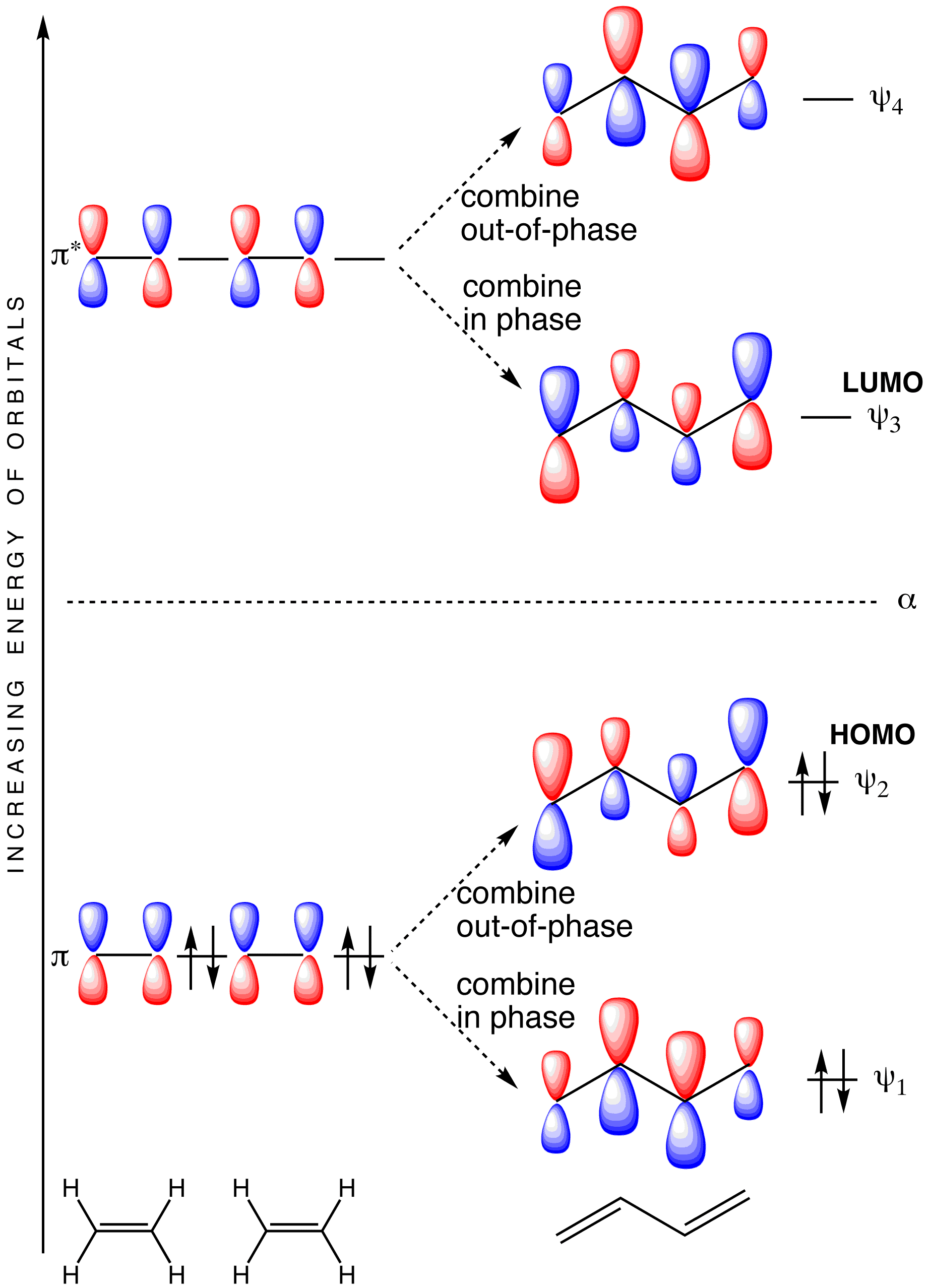
Molecular Orbital Diagram of Ethene Through a Fragment Molecular ... In this video we will generate a qualitative MO diagram of ethene through a fragment molecular orbital approach. This will be done by combining two methylene fragments together to generate... Pi Molecular Orbitals 1,3,5 Hexatriene - Chad's Prep® 1,3,5-Hexatriene Molecular Orbital Diagram Pi Molecular Orbitals of 1,3,5-Hexatriene With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure. C2H2 Lewis Structure, Molecular Geometry ... - Techiescientist Step 1: Search for the total number of valence electrons one molecule of acetylene already has: It is 10 for a single acetylene (C2H2) molecule. Step 2: Search for how many more valence electrons one molecule of acetylene requires: It is 10 for a single acetylene (C2H2) molecule. Step 3: Find the central atom to begin drawing the structure: As ...
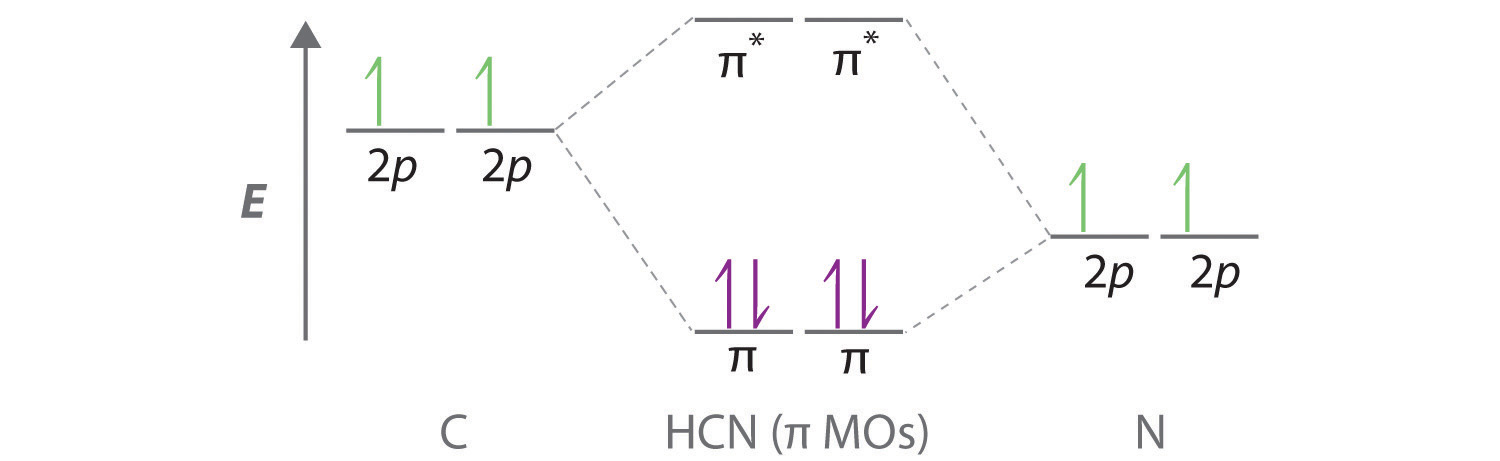
Ethylene molecular orbital diagram. Ethylene - Thermophysical Properties - Engineering ToolBox Ethylene - Thermophysical Properties. Chemical, physical and thermal properties of ethylene, also called ethene, acetene and olefiant gas. Phase diagram included. Ethylene, C2H4, is a highly flammable, colorless and noncorrosive gas with a sweet odor. It is easily ignited and a flame can easily flash back to the source of the leak. Molecular orbitals in ethylene - Big Chemical Encyclopedia In ethylene, both the HOMO and LUMO are formed primarily from p orbitals from the two carbons. The carbons lie in the YZ-plane, and so the p,j orbitals lie above and below the C-C bond. In the HOMO, the orbitals have like signs, and so they combine to form a bonding n molecular orbital. Solved a) Considering the molecular orbital diagram for - Chegg See Answer. a) Considering the molecular orbital diagram for ethylene above, draw the molecules (with some kind of three-dimensional representation) of the initial interaction of ethylene with a very strong acid (represent this as H-A). b) Finish the molecular orbital diagram of formaldehyde (H 2 CO) by making the molecular orbitals in the middle. Frontier molecular orbital theory - Wikipedia Theory [ edit] Fukui realized that a good approximation for reactivity could be found by looking at the frontier orbitals ( HOMO/LUMO ). This was based on three main observations of molecular orbital theory as two molecules interact: The occupied orbitals of different molecules repel each other. Positive charges of one molecule attract the ...
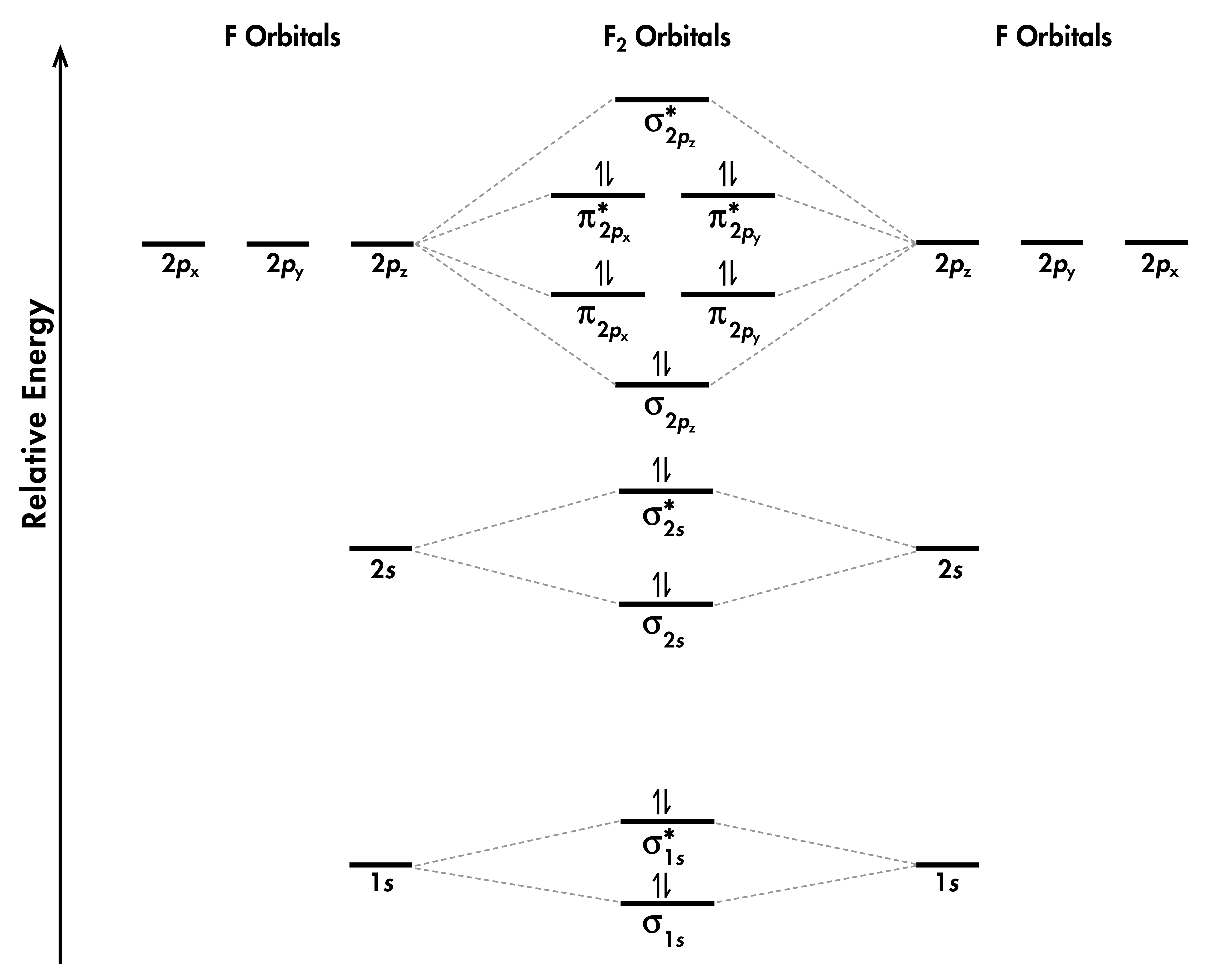
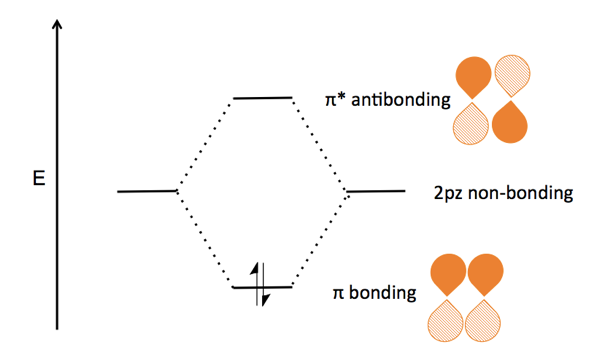
Hybridization: Structure of Ethylene | MCC Organic Chemistry The correct Lewis structure for ethene is shown below: In the molecule ethene, both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital. These p-orbitals will undergo parallel overlap and form one σ σ bond with bean-shaped probability areas above and below the plane of the six atoms. 13.2. Molecular orbitals for ethene | Organic Chemistry II Molecular orbitals for ethene (ethylene) In the bonding pi orbital, the two shaded lobes of the p orbitals interact constructively with each other, as do the two unshaded lobes (remember, the arbitrary shading choice represents mathematical (+) and (-) signs for the mathematical wavefunction describing the orbital). Ethylene, molecular orbitals - Big Chemical Encyclopedia In ethylene, both the HOMO and LUMO are formed primarily from p orbitals from the two carbons. The carbons lie in the YZ-plane, and so the p,j orbitals lie above and below the C-C bond. In the HOMO, the orbitals have like signs, and so they combine to form a bonding n molecular orbital. Answered: Determine the molecular orbital… | bartleby A: Using the molecular orbital diagram of O2+ calculate the bond order of O2+ ? Q: Fill in the molecular orbital energy diagram for the diatomic molecule C2. carbong MO's carbona 2p… A: A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical…
Molecular orbital diagrams of methylene | Download Scientific Diagram Download scientific diagram | Molecular orbital diagrams of methylene from publication: Photogenerated reactive intermediates from thiophene ylides: thiophenes, oxenes and carbenes, oh my ... Ethene (ethylene): Molecular Geometry - Hybridization - Molecular ... Lewis structure: A Lewis structure or Lewis representation (also known as electron raster diagram, Lewis raster formula, Lewis point structure, or point electron structure) is a two-dimensional diagram used in chemistry to show the bonding between atoms of a molecule and the lone electron pairs that may be present in this molecule. Molecular Orbital theory: Ethylene - Blogger Molecular Orbital theory Monday, 3 November 2014 Ethylene • Standard bonding picture for ethylene is viewed as being made from 2 sp2 hybridized C atoms, and consists of a C-C double bond. • MOT does not employ hybridization and does not assume bonding arrangements. • Build ethylene from two CH2 groups without preconceived bonding arrangements. C2H4 Lewis Structure, Molecular Geometry ... - Techiescientist The other sp2 hybrid orbitals form sigma bonds between C and H, therefore, leading to C-H single bonding structure. C2H4 Molecular Orbital (MO) Diagram. The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles.
13.2: Molecular orbitals for ethene - Chemistry LibreTexts Molecular orbitals for ethene (ethylene) In the bonding pi orbital, the two shaded lobes of the p orbitals interact constructively with each other, as do the two unshaded lobes (remember, the arbitrary shading choice represents mathematical (+) and (-) signs for the mathematical wavefunction describing the orbital).
Ch 10: Ethene MOs In chapter 1 we saw that the molecular orbitals of H2are created by the combination of 1s orbitals. The in-phase combination gave the bonding orbital. The out-of-phase combination the anti-bonding orbital. For ethene, the σ framework is created by the interaction of the sp2hybrid orbitals of the C atoms and H1s orbitals.
Molecular Orbital Diagram Of Ethene Each line in this diagram represents one pair of shared electrons. Ethene This sideways overlap also creates a molecular orbital, but of a different kind. In this. Ethylene is the simplest molecule that has a double bond. As we saw from the valence bond model, we should find the presence of a σ-bond framework, and a .
Ethylene | CH2=CH2 - PubChem Ethylene | CH2=CH2 or C2H4 | CID 6325 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ...
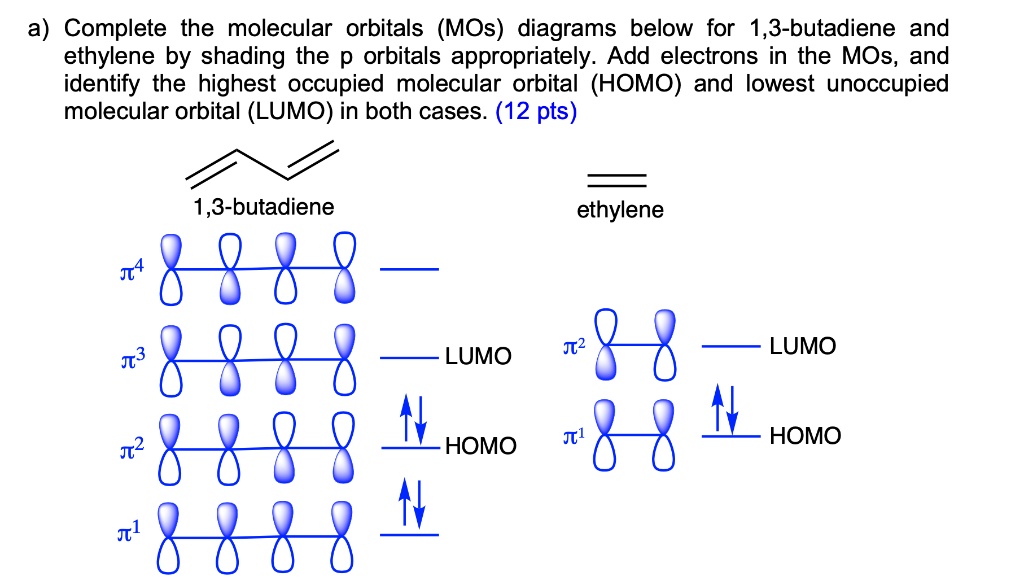
Molecular Orbitals in the Diels-Alder Reaction - UC Santa Barbara Table below shows π molecular orbitals for ethylene (dienophile) and 1,3-butadiene; clicking on the image will bring up Virtual Reality Modeling Language models for orbitals. Outside Resources Diels-Alder Reaction by Francis A. Carey at University of Calgary Diels-Alder Reaction at
π- Molecular Orbitals (MO's) of Ethylene - Yale University π- Molecular Orbitals (MO's) of Ethylene (The appearance of molecular orbitals may take a few moments.) How to Manipulate JSmol Structures or Click on the JSmol logo.. For a discussion of molecular orbitals, click here.
PDF Molecular Orbitals: Example 1: Ethylene Looking only at the π orbitals: - heat An electron can be excited from the HOMO to the LUMO using light of a precise wavelength dependent on the energy difference between the two orbitals (since the orbitals are quantized). The electron can go back to it's original orbital and heat (or light) is produced in the process. Example 2: 1,3 ...
Ethylene molecular orbitals PowerPoint (PPT) Presentations, Ethylene ... Bonding Theory: 1. molecular orbitals: When two atoms combine, the molecular orbital model assumes that their atomic orbitals overlap to produce orbitals that apply to the entire molecule. a. belongs to a molecule as a whole.
Bonding orbitals in Ethylene (Ethene) sp2 - ChemTube3D Bonding orbitals in Ethylene (Ethene) sp 2 CONTROLS Use the buttons to display the sp 2 orbitals that make up the sigma framework and the remaining p orbitals which form the pi-bond. Explore bonding orbitals in other small molecules
Ethylene - Wikipedia Orbital description of bonding between ethylene and a transition metal. This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon.
C2H2 Lewis Structure, Molecular Geometry ... - Techiescientist Step 1: Search for the total number of valence electrons one molecule of acetylene already has: It is 10 for a single acetylene (C2H2) molecule. Step 2: Search for how many more valence electrons one molecule of acetylene requires: It is 10 for a single acetylene (C2H2) molecule. Step 3: Find the central atom to begin drawing the structure: As ...
Pi Molecular Orbitals 1,3,5 Hexatriene - Chad's Prep® 1,3,5-Hexatriene Molecular Orbital Diagram Pi Molecular Orbitals of 1,3,5-Hexatriene With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure.
Molecular Orbital Diagram of Ethene Through a Fragment Molecular ... In this video we will generate a qualitative MO diagram of ethene through a fragment molecular orbital approach. This will be done by combining two methylene fragments together to generate...


















0 Response to "39 ethylene molecular orbital diagram"
Post a Comment